Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Hindrance of photodimerization of coumarin derivative induced by pillar[5]arene-based molecular recognition in water†
Chunwen
Yang
,
Haixiong
Shi
,
Shanshan
Li
and
Qiao
Li
 *
*
College of Chemical Engineering, Lanzhou University of Arts and Science, Lanzhou, Gansu 730000, P. R. China. E-mail: gansuchengzhou@126.com; Fax: +86 9318685568; Tel: +86 9318685568
First published on 5th January 2017
Abstract
In this paper, an unprecedented method for hindrance of the photodimerization of coumarin derivative induced by pillar[5]arene-based molecular recognition in water is reported.
Photophysical and photochemical behavior of coumarin derivatives have attracted much interest by scientists in recent years.1 After ultraviolet (UV) irradiation at wavelengths greater than 310 nm, a coumarin moiety will undergo dimerization through [2π + 2π] cycloaddition.2 In addition, coumarin and its derivatives have been widely used in designing photosensitive polymeric materials and have been investigated for use as lasers and antenna models of artificial photosynthesis because of their high quantum yield. For example, Zhang et al. reported a photo-controllable supramolecular gel, which is achieved by incorporating photoactive coumarin moieties into a tribranched monomer and then self-assembly with cyclodextrin (CD) followed by noncovalent–covalent switchover.3 Although there are so many applications relating to the photodimerization of coumarin derivatives, studies of the hindrance of photodimerization of coumarin derivatives have rarely been reported. This greatly impedes the development of applications of coumarin derivatives. Therefore, studies of the hindrance of photodimerization of coumarin derivatives are needed.
Pillar[n]arenes are a new class of supramolecular hosts similar to crown ethers,4 CDs,5 calixarenes6 and cucurbiturils7 and they were discovered in 2008.8 Their syntheses, functionalizations, host–guest properties, conformations and the applications in different fields have been actively studied.9 Their unique structure and easy functionalization properties give them superior properties in host–guest recognition. However, most of these studies have been focused on the cyclic dimers, supramolecular polymers, chemosensors, drug delivery systems, transmembrane channels and use as a cell glue. In sharp contrast, only a few efforts have been made to explore their application in the control of organic reactions. The lack of such applications may greatly impede the use of pillararenes in the field of supramolecular chemistry. Therefore, it is important and necessary to explore the application of the control of organic reactions based on pillararene-based molecular recognition. Thus, the unprecedented hindrance of the photodimerization of coumarin induced by pillar[5]arene-based molecular recognition in water is reported in this paper. Furthermore, this host–guest system can successfully be used in supra-amphiphile self-assembly in water. In particular, this is the first time that pillararene-based molecular recognition was used for the hindrance of the photodimerization of coumarin and which was then used further for supra-amphiphile self-assembly in water.
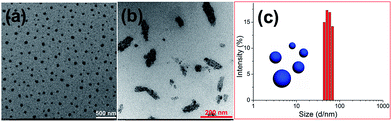
Firstly, a water-soluble coumarin derivative (G, Scheme 1) was designed and synthesized, which contained one trimethylammonium group and a coumarin unit. Because G contains a coumarin group, the possibility of whether G could undergo dimerization through [2π + 2π] cycloaddition under UV light (365 nm) in water was investigated. In order to confirm this, the photodimerization behavior was first studied using proton nuclear magnetic resonance (1H-NMR) spectroscopy. According to the 1H-NMR spectrum of an aqueous solution of G, the photodimerization can be observed after UV irradiation (365 nm, 4.0 h) in water on the 1H-NMR timescale (Fig. 1). Peaks related to protons H1–H2 on G, were shifted upfield after UV irradiation. Meanwhile, peaks related to protons H3–H5 on G were also shifted upfield. These results indicated that G could undergo dimerization through [2π + 2π] cycloaddition under UV light (365 nm) in water. In addition, the self-assembly behavior of molecule G before and after UV irradiation in water was also investigated. Amphiphilic G self-assembles into nanoparticles in water before UV irradiation (Scheme 1). After irradiation under 365 nm UV light for 4.0 h, these nanoparticles will transform into nanosheets because of the photodimerization of molecule G (Scheme 1). The conductivities of the solutions as functions of the concentrations of G before and after UV irradiation were measured to determine the critical aggregation concentrations (CAC). The two linear segments in the curve and a sudden reduction of the slope indicate that the CAC value of G before UV irradiation is approximately 1.45 × 10−4 M (Fig. S6a, ESI†) and the CAC value of G after UV irradiation is approximately 1.72 × 10−4 M (Fig. S6b, ESI†). The self-assembly behaviour of G before and after UV irradiation was subsequently investigated in water using transmission electron microscopy (TEM). TEM experiments helped in the visualization of the self-assembled nanostructures from G before and after UV irradiation. Fig. 2a shows TEM micrographs of G aggregates before UV irradiation. Spherical assemblies of about 50 nm in diameter were obtained before UV irradiation and sheet assemblies were obtained after UV irradiation (365 nm, 4.0 h) (Fig. 2b). The dynamic light scattering (DLS) result (Fig. 2c) showed that the aggregates of G before UV irradiation have an average diameter of ∼50 nm with a narrow size distribution, which supports the TEM result.
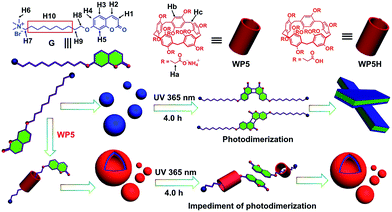 | ||
| Scheme 1 Schematic representation of the hindrance of photodimerization and the self-assembly process of G and WP5⊃G in water. | ||
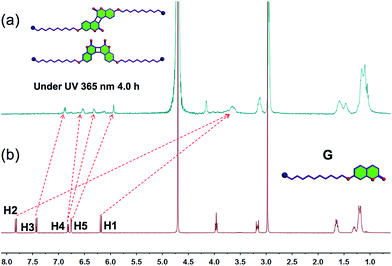 | ||
| Fig. 1 1H-NMR spectra (400 MHz, deuterium oxide [D2O], 298 K) of G (10.0 M): (a) before UV irradiation; (b) after UV irradiation for 4.0 h (365 nm). | ||
It has been well established that pillararenes can complex with positively charged guests.10 Because G contains a trimethylammonium group, an investigation was carried out to determine whether G could complex with WP5 to form a pseudorotaxane and further hinder the photodimerization of G. In order to confirm this, the host–guest complexation between WP5 and G was first studied using 1H-NMR. According to the 1H-NMR spectrum of an equimolar (10.0 mM) aqueous solution of WP5 and G, the complexation rapidly exchanges on the 1H-NMR timescale (Fig. 3). Peaks related to protons H8–H10 on G shifted upfield after complexation. As can be seen in Fig. 3, the chemical shift of H9 slightly shifted upfield while that of H8 shifted greatly upfield. This is abnormal because H9 is closer to WP5 than H8. Meanwhile, peaks related to protons Ha–c on WP5 shifted downfield. Furthermore, a two-dimensional (2D) nuclear Overhauser effect spectroscopy (NOESY) NMR study of the aqueous solution of G and WP5 was performed to investigate the relative spatial positions of protons in this host–guest complex (Fig. S7, ESI†). Correlation signals were observed between protons H8–H10 on G and protons Ha–c on WP5. These results indicated that the positively charged trimethylammonium head of G was threaded into the cavity of the cyclic host WP5 to form a pseudorotaxane.
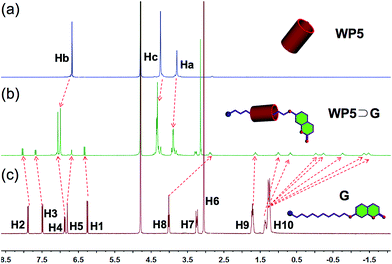 | ||
| Fig. 3 Partial 1H-NMR (400 Hz, D2O, 293 K) spectra: (a) WP5 (10.0 mM); (b) WP5 (10.0 mM) and G (10.0 mM); (c) guest molecule G (10.0 mM). | ||
After the WP5⊃G recognition motif was established, an investigation was carried to determine whether the photodimerization behavior of G could be hindered by the host–guest interaction between WP5 and G in water. Several experiments were performed to confirm that the hindrance of photodimerization of G was induced by pillar[5]arene-based molecular recognition in water. Firstly, the hindrance of photodimerization behavior induced by pillar[5]arene-based molecular recognition was studied using 1H-NMR. As shown in Fig. 4, after the UV irradiation (365 nm) for 4.0 h, peaks related to protons on G showed no changes. So, the photodimerization behavior of the guest molecule G can be successfully hindered by the host–guest interaction between WP5 and G in water. According to the results shown in Fig. 3, 4 and S7 (ESIref E \* MERGEFORMAT †), because H9 and H8 are closed to WP5 in the WP5⊃G complex, the steric hindrance of WP5⊃G can successfully hinder the photodimerization behavior of the guest molecule G.
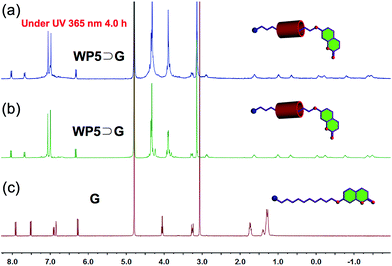 | ||
| Fig. 4 1H-NMR (400 Hz, D2O, 293 K) spectra: (a) WP5⊃G (10.0 mM) after UV irradiation 4.0 h (365 nm) (10.0 mM); (b) WP5⊃G (10.0 mM) before UV irradiation (10.0 mM); (c) guest molecule G (10.0 mM). | ||
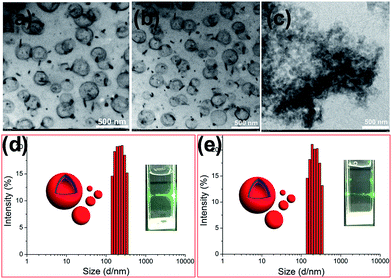
Furthermore, the self-assembly behavior of the host–guest complex WP5⊃G before and after UV irradiation was investigated in water. The conductivities of the solutions as a function of the concentrations of WP5⊃G before and after UV irradiation were measured to determine the CAC. The two linear segments in the curve and a sudden reduction of the slope indicate that the CAC value of WP5⊃G before UV irradiation was approximately 5.2 × 10−5 M (Fig. S8a, ESI†) and the CAC value of WP5⊃G after UV irradiation was approximately 5.0 × 10−5 M (Fig. S8b, ESI†). There is no obvious changes of the CAC value of WP5⊃G before and after UV irradiation. The self-assembly behaviour of WP5⊃G before and after UV irradiation was subsequently investigated in water using TEM. The results of the TEM experiments helped in the visualization of the self-assembled nanostructures from WP5⊃G before and after UV irradiation. Fig. 5 shows TEM micrographs of WP5⊃G aggregates before and after UV irradiation. Vesicles of about 200 nm in diameter were obtained with WP5⊃G both before and after UV irradiation (365 nm, 4.0 h). Tyndall effects (Fig. 5d and e) were observed for the solutions of WP5⊃G before and after UV irradiation, indicating the formation of the nanostructures of WP5⊃G before and after UV irradiation. DLS results (Fig. 5d and e) showed that the aggregates of WP5⊃G before and after UV irradiation have an average diameter of 200 nm with a narrow size distribution, which supports the TEM results. These results provided direct evidence that the photodimerization behavior of the guest molecule G was successfully hindered by the host–guest interaction between WP5 and G in water.
From previous work,11 it was known that the complex WP5⊃G can be easily destroyed by acid, because acid protonates the carboxylate groups to convert WP5 to WP5H (Scheme 1), resulting in WP5H precipitation from the aqueous solution. So, when the pH of the aqueous solution of WP5 and G decreased to 2.00, the self-assembly morphology of WP5⊃G changed from nanoparticles to irregular aggregates because the complex WP5⊃G was destroyed (Fig. 5c). These results indicated that this supramolecular amphiphile had pH responsiveness.
In conclusion, a previously unknown method for the hindrance of the photodimerization of coumarin induced by pillar[5]arene-based molecular recognition in water has been found. Several experiments were performed to confirm the hindrance of photodimerization of G induced by pillar[5]arene-based molecular recognition in water. Furthermore, this host–guest system can be successfully used in supra-amphiphile self-assembly in water. Amphiphilic G self-assembles into nanoparticles in water before UV irradiation. After irradiation under 365 nm UV light for 4.0 h, these nanoparticles will transform into nanosheets because of the photodimerization of molecule G. Furthermore, the host–guest complex WP5⊃G can self-assemble into vesicles before UV irradiation. Because of the hindrance of photodimerization of G induced by pillar[5]arene-based molecular recognition, the host–guest complex WP5⊃G can still self-assemble into vesicles after UV irradiation (365 nm, 4.0 h). And, this host–guest complex WP5⊃G has very good pH responsiveness. Finally, the present study provided a new and simple method to hinder the photodimerization of the coumarin derivative, which may be of high importance for the development of novel functional materials and molecular devices in the future.
Acknowledgements
This work was supported by the Natural Science Foundation of Gansu (No. 1010RJZA196).Notes and references
- (a) G. S. Hammond, C. A. Stout and A. A. Lamola, J. Am. Chem. Soc., 1964, 86, 3103 CrossRef CAS; (b) K. Muthuramu and V. Ramamurthy, J. Org. Chem., 1982, 47, 3976 CrossRef CAS; (c) X. Yu, D. Scheller, O. Rademacher and T. Wolff, J. Org. Chem., 2003, 68, 7386 CrossRef CAS PubMed; (d) L. H. Leenders, E. Schouteden and F. C. De, J. Org. Chem., 1973, 38, 957 CrossRef CAS; (e) H. A. Morrison, H. Curtis and T. McDowell, J. Am. Chem. Soc., 1966, 88, 5415 CrossRef CAS.
- (a) T. Wolff and H. Goerner, Phys. Chem. Chem. Phys., 2004, 6, 368 RSC; (b) K. Gnanaguru, N. Ramasubbu, K. Venkatesan and V. Ramamurthy, J. Org. Chem., 1985, 50, 2337 CrossRef CAS; (c) K. Vishnumurthy, T. N. G. Row and K. Venkatesan, Tetrahedron, 1998, 54, 11235 CrossRef CAS.
- Q. Zhang, D.-H. Qu, X. Ma and H. Tian, Chem. Commun., 2013, 49, 9800 RSC.
- (a) F. Huang and H.-W. Gibson, Chem. Commun., 2005, 13, 1696 RSC; (b) Y.-S. Su, J.-W. Liu, Y. Jiang and C.-F. Chen, Chem.–Eur. J., 2011, 17, 2435 CrossRef CAS; (c) S. Li, G. Weng, W. Lin, Z. Sun, M. Zhou, B. Zhu, Y. Yea and J. Wu, Polym. Chem., 2014, 5, 3994 RSC; (d) Z. Niu, F. Huang and H.-W. Gibson, J. Am. Chem. Soc., 2011, 133, 2836 CrossRef CAS PubMed.
- A. Harada, Y. Takashima and H. Yamaguchi, Chem. Soc. Rev., 2009, 38, 875 RSC.
- D.-S. Guo and Y. Liu, Chem. Soc. Rev., 2012, 41, 5907 RSC.
- (a) J. M. Zayed, N. Nouvel, U. Rauwald and O. A. Scherman, Chem. Soc. Rev., 2010, 39, 2806 RSC; (b) K. M. Park, K. Suh, H. Jung, D.-W. Lee, Y. Ahn, J. Kim, K. Baeka and K. Kim, Chem. Commun., 2009, 71 RSC; (c) H. Yang, Y. Bai, B. Yu, Z. Wang and X. Zhang, Polym. Chem., 2014, 5, 6439 RSC.
- T. Ogoshi, S. Kanai, S. Fujinami, T. Yamagishi and Y. Nakamoto, J. Am. Chem. Soc., 2008, 130, 5022 CrossRef CAS PubMed.
- (a) D.-R. Cao, Y.-H. Kou, J.-Q. Liang, Z.-Z. Chen, L.-Y. Wang and H. Meier, Angew. Chem., Int. Ed., 2009, 48, 9721 CrossRef CAS; (b) L. Chen, Z. Li, Z. Chen and J.-L. Hou, Org. Biomol. Chem., 2013, 11, 248 RSC; (c) X. B. Hu, Z.-X. Chen, G.-F. Tang, J.-L. Hou and Z.-T. Li, J. Am. Chem. Soc., 2012, 134, 8384 CrossRef CAS PubMed; (d) G. Yu, M. Xue, Z. Zhang, J. Li, C. Han and F. Huang, J. Am. Chem. Soc., 2012, 134, 13248 CrossRef CAS; (e) M. Holler, N. Allenbach, J. Sonet and J.-F. Nierengarten, Chem. Commun., 2012, 48, 2576 RSC; (f) G. Yu, C. Han, Z. Zhang, J. Chen, X. Yan, B. Zheng, S. Liu and F. Huang, J. Am. Chem. Soc., 2012, 134, 8711 CrossRef CAS; (g) C. Li, J. Ma, L. Zhao, Y. Zhang, Y. Yu, X. Shu, J. Li and X. Jia, Chem. Commun., 2013, 49, 1924 RSC; (h) H. Li, D.-X. Chen, Y.-L. Su, Y.-B. Zheng, L.-L. Tan, P.-S. Weiss and Y.-W. Yang, J. Am. Chem. Soc., 2013, 135, 1570 CrossRef CAS; (i) X. Wang, H. Deng, J. Li, K. Zheng, X. Jia and C. Li, Macromol. Rapid Commun., 2013, 34, 1856 CrossRef CAS; (j) J.-F. Xu, Y.-Z. Chen, L.-Z. Wu, C.-H. Tung and Q. Yang, Org. Lett., 2013, 15, 6148 CrossRef CAS; (k) R.-R. Kothur, J. Hall, B.-A. Patel, C.-L. Leong, M.-G. Boutelle and P.-J. Cragg, Chem. Commun., 2014, 50, 852 RSC; (l) L. Chen, Z. Li, Z. Chen and J.-L. Hou, Org. Biomol. Chem., 2013, 11, 248 RSC; (m) L. Wu, Y. Fang, Y. Jia, Y. Yang, J. Liao, N. Liu, X. Yang, W. Feng, J. Ming and L. Yuan, Dalton Trans., 2014, 43, 3835 RSC; (n) Y. Wang, J. Xu, Y. Chen, L. Niu, L. Wu, C. Tung and Q. Yang, Chem. Commun., 2014, 50, 7001 RSC; (o) X. Shu, W. Chen, D. Hou, Q. Meng, R. Zheng and C. Li, Chem. Commun., 2014, 50, 4820 RSC; (p) J. Zhou, M. Chena and G. Diao, Chem. Commun., 2014, 50, 11954 RSC; (q) C. Li, K. Han, J. Li, Y. Zhang, W. Chen, Y. Yu and X. Jia, Chem.–Eur. J., 2013, 19, 11892 CrossRef CAS PubMed; (r) W. Hu, H. Yang, W. Hu, M. Ma, X. Zhao, X. Mi, Y. Liu, J. Li, B. Jiang and K. Wen, Chem. Commun., 2014, 50, 10460 RSC; (s) C.-L. Sun, J.-F. Xu, Y.-Z. Chen, L.-Z. Wu, C.-H. Tung and Q.-Z. Yang, Polym. Chem., 2016, 7, 2057 RSC.
- (a) M. Xue, Y. Yang, X. Chi, Z. Zhang and F. Huang, Acc. Chem. Res., 2012, 45, 1294 CrossRef CAS; (b) B. Shi, K. Jie, Y. Zhou, D. Xia, J. Zhou and F. Huang, J. Am. Chem. Soc., 2016, 138, 80 CrossRef CAS.
- (a) Y. Yao, X. Chi, Y. Zhou and F. Huang, Chem. Sci., 2014, 5, 2778 RSC; (b) L.-B. Meng, W. Zhang, D. Li, Y. Li, X.-Y. Hu, L. Wang and G. Li, Chem. Commun., 2015, 51, 14381 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures, characterizations and other materials. See DOI: 10.1039/c6ra26741f |
| This journal is © The Royal Society of Chemistry 2017 |