Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A novel H2O2 responsive supramolecular hydrogel for controllable drug release†
Chunhua
Ren
,
Liping
Chu
,
Fan
Huang
,
Lijun
Yang
,
Huirong
Fan
*,
Jianfeng
Liu
and
Cuihong
Yang
 *
*
Tianjin Key Laboratory of Radiation Medicine and Molecular Nuclear Medicine, Institute of Radiation Medicine, Chinese Academy of Medical Science and Peking Union Medical College, Tianjin, 300192, China. E-mail: yangcuihong@irm-cams.ac.cn
First published on 5th January 2017
Abstract
Due to the important significance of hydrogen peroxide (H2O2) in physiology, aging and disease in living organisms, tremendous effort has been devoted to develop H2O2 responsive materials for the detection of its over production or for controlled drug release. However, it is still challenging to develop H2O2 responsive supramolecular hydrogels. In this study, we designed and synthesized a novel H2O2 responsive peptide hydrogelator bearing the thiazolidinone group. A supramolecular hydrogel based on peptide self-assembly was prepared through a heating–cooling process and its gel–sol phase transition could be triggered by the removal of thiazolidinone groups upon H2O2 oxidization. The excellent H2O2 responsive property of the supramolecular hydrogel was investigated by LC-MS, rheology and TEM. A drug release study in vitro demonstrated that the gel–sol phase transition could be applied for releasing gemcitabine sustainedly and controllably. Our study could provide a new way for the design of H2O2 responsive materials and hold great potential in the application of anticancer drug delivery.
Peptide-based supramolecular hydrogels have attracted extensive research interest in the past few decades due to their inherent advantages, such as ease of design and synthesis, good biocompatibility and degradability.1 Since supramolecular hydrogels are formed by non-covalent interactions (hydrogen bond, π–π, hydrophobic, and charge interactions), they are therefore extremely sensitive to external stimuli, including pH,2 light,3 enzymes,4 ions,5 and redox agents.6 Increasing numbers of intelligent responsive supramolecular hydrogels have been developed so far, and they have shown great promise in the applications of drug delivery,7 cancer cell inhibition,8 vaccine adjuvants9 and detection of important analytes.10
Hydrogen peroxide (H2O2), as a second messenger for intracellular signal transduction,11 usually results from cellular metabolism of molecular oxygen. In most cases, it is maintained at well-balanced concentration levels and plays crucial roles in cell proliferation, cell differentiation and cell migration.12 The over-production of H2O2 can lead to high oxidative stress and therefore impaired cellular structures.13 Many reports have demonstrated that a series of pathologies are associated with elevated levels of H2O2 including inflammation,14 cancer,15 cardiovascular disorders,16 and neurodegenerative diseases.17 Consequently, enormous efforts have been made to develop H2O2 responsive nano-systems for controlled drug release to treat these diseases or for the detection of its over-production. These nano-systems are mostly based on the conventional peroxalate ester that can react with H2O2. For example, Murthy and co-workers have reported on nanoparticles formulated from peroxalate and fluorescent dyes capable of imaging hydrogen peroxide in vivo with high specificity and sensitivity.18 There are also several reports regarding H2O2 responsive supramolecular hydrogels. For example, Hamachi and co-workers have recently reported on responsive peptide-based hydrogels containing a H2O2-reactive boronoarylmethoxycarbonyl group and capable of retaining the activity of encapsulated enzymes.19 They have demonstrated that the programmable hybridization of the hydrogel and oxidases enables the resulting materials responding to not only H2O2 molecules but also a variety of disease-related biomarkers. These pioneering works highlight the importance of H2O2 responsive materials including the supramolecular hydrogels.
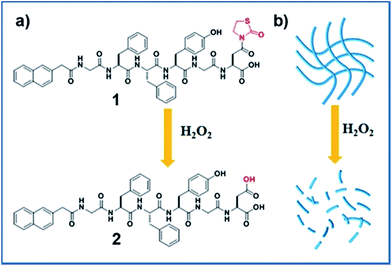
Recently, a novel H2O2 responsive group, thiazolidinone modified carboxylic acid, has been reported, and it can release free carboxylic acid upon the activation of H2O2.20 Inspired by this work, we opted to develop a novel H2O2 responsive supramolecular hydrogelator bearing the thiazolidinone group. We therefore designed a short peptide derivative Nap-GFFYGD(Thi) (compound 1) bearing a H2O2 responsive thiazolidinone at the C-terminal of the peptide. As shown in Scheme 1, compound 1 was expected to form nanofibers and a hydrogel by supramolecular self-assembly at a given concentration. The removal of thiazolidinone through the oxidation/elimination reaction by H2O2 was expected to produce a more hydrophilic peptide Nap-GFFYGD (2), resulting the dis-assembly of nanofibres and a gel–sol transition. The synthesis route and purification process of Fmoc-aspartic acid decorated with thiazolidinone (D(Thi)) were described in Scheme S1.† We then prepared compound 1 by standard solid phase peptide synthesis (SPPS) and obtained the pure compound by reverse-phase high performance liquid chromatography (HPLC).
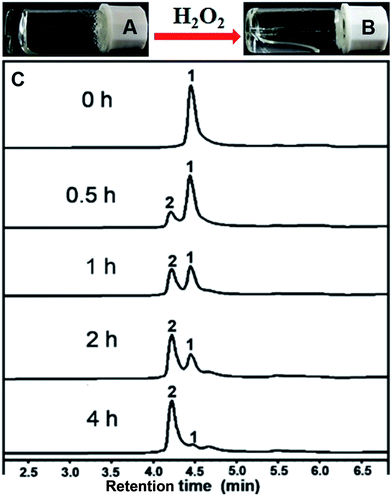
After obtaining the designed compound, the gelation property of compound 1 was firstly examined. Results in Fig. 1A indicated that it could form a hydrogel at a minimum gelation concentration (MGC) of 0.1 wt% within 5 minutes upon a heating–cooling process. We then tested the H2O2 responsive property of the hydrogel. A gel–sol transformation was observed after adding H2O2 (40 mM) to the gel at 37 °C for 4 hours (Fig. 1B). H2O2-response sensitivity of the hydrogel was evaluated through the gel–sol transition after the addition of 0–100 mM H2O2 to the gel. The results showed that at least 4 mM H2O2 was required to induce a complete collapse of the gel after 24 hours (Fig. S9†) and the time required for the gel–sol transition ranged from 1.5 to 24 hours depending on the amount of H2O2 (Fig. S10†). Liquid chromatography mass spectrometer (LC-MS) was employed to analyse the gel–sol phase transition. As shown in Fig. 1C, compound 1 gradually converted to compound 2 after the addition of H2O2. For instance, the conversion percentage was about 50% at one hour time point and reached an equilibrium after about four hours with a conversion rate of about 96% (Fig. S11†). The mass spectra of resulting solution in Fig. 1B (Fig. S7†) indicated the molecular weight peak of Nap-GFFYGD (2). These observations clearly demonstrated the success of our design, and the H2O2 responsive property of our hydrogel suggested its potential application in controlled drug release.
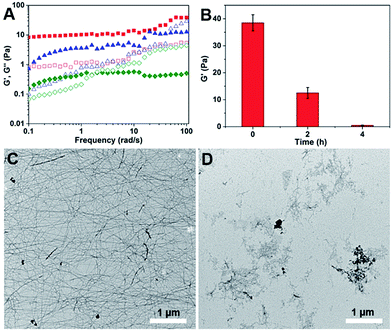
Rheology was also performed to characterize the mechanical properties of the hydrogels before and after the addition of H2O2. The dynamic frequency sweep was carried at a fixed strain value of 0.5% in the frequency range from 0.1 to 100 rad s−1. As shown in Fig. 2A and S12,† before the addition of H2O2, the G′ value of the hydrogel was an order of magnitude bigger than its G′′ value in the tested frequency range, indicating a true hydrogel formation.21 After incubation with H2O2 for two hours and four hours, the G′′ value of the samples become bigger than their corresponding G′ value at frequency value of 50 and 2 rad s−1, respectively. At the same time, the G′ value decreased dramatically from 40 Pa to 0.4 Pa after four hours' incubation with H2O2 (Fig. 2B). These observations suggested the formation of viscous solutions after the addition of H2O2. Transmission electron microscopy (TEM) was then used to characterize the morphology of nanostructures in the hydrogel and the obtained solution. As shown in Fig. 2C, uniform nanofibers were observed in the hydrogel with the diameter of about 25 nm and the length of up to several microns. They entangled with each other to form dense networks for the hydrogel formations. Upon the addition of H2O2 overnight, the long nanofibers disappeared and only few short nanofibers could be observed (Fig. 2D).
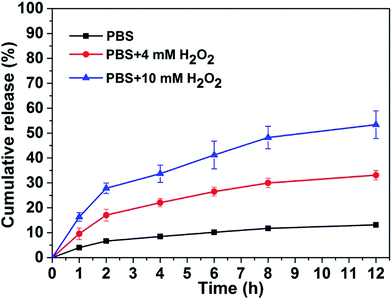
We therefore tested the possible application of our responsive hydrogel in controlled drug release. We choose gemcitabine as a drug molecule to investigate its controlled release property from our hydrogel at 37 °C. A 0.25 mL of PBS solution with different amounts of H2O2 was placed on top of the gel (0.2 mL). The upper solution was totally taken out at different time intervals following by adding another 0.25 mL fresh PBS solution containing corresponding H2O2. The accumulation percentage of released gemcitabine from the hydrogel were quantified by LC-MS based on a standard curve. Results in Fig. 3 demonstrated that the release speed of gemcitabine depended on the amount of H2O2 in PBS solution. That was, the more H2O2 in PBS solution, the faster gemcitabine released from the hydrogel. In the absence of H2O2, the accumulative release percentage of gemcitabine was only about 10% in 12 hours. While in the presence of 4 and 10 mM H2O2, the accumulative release percentage of gemcitabine from the gel was about 30% and 50%, respectively in 12 hours. Since cancer cells exhibit elevated levels of H2O2 compared with normal cells,22 our H2O2 responsive peptide hydrogel might be used as a potential delivery system for anticancer drug delivery to tumor sites.
In summary, we have developed a novel supramolecular hydrogel based on peptide self-assembly with a gel–sol phase transition triggered by H2O2. The hydrogel showed an excellent H2O2 responsive property and could release gemcitabine sustainedly and controllably. Compared with conventional H2O2 responsive materials involving peroxalate ester or boronoaryl groups, the synthesis of thiazolidinone modified hydrogelator were relatively easier and more straightforward, and the gelator could avoid being cleaved by ubiquitous esterases in vivo. However, due to the limited structure change of thiazolidinone modified hydrogelator upon H2O2 oxidization, the H2O2-response sensitivity of the hydrogelator developed by us was not as high as that of peroxalate ester and boronoaryl groups and should be further improved in the future study. As the most important marker for reactive oxygen species (ROS), H2O2 is increasingly investigated in physiology, aging and disease in living organisms. Our supramolecular hydrogel system could provide a new way for the detection of the overproduced H2O2 and hold great potential in the application of anticancer drug delivery.
Acknowledgements
This work is supported by National Natural Science Foundation of China (No. 81471727), Outstanding Young Faculty Award of Peking Union Medical College (YR1579), PUMC Youth Fund and the Fundamental Research Funds for the Central Universities (3332015100), Fundamental Research Funds for CAMS & PUMC (2016ZX310082) and CAMS Initiative for Innovative Medicine (2016-I2M-3-022).Notes and references
- J. H. Collier, J. S. Rudra, J. Z. Gasiorowski and J. P. Jung, Chem. Soc. Rev., 2010, 39, 3413–3424 RSC; S. Toledano, R. J. Williams, V. Jayawarna and R. V. Ulijn, J. Am. Chem. Soc., 2006, 128, 1070–1071 CrossRef CAS PubMed; X. Zhao and S. Zhang, Chem. Soc. Rev., 2006, 35, 1105–1110 RSC; S. Fleming and R. V. Ulijn, Chem. Soc. Rev., 2014, 43, 8150–8177 RSC; J. W. Steed, Chem. Commun., 2011, 47, 1379–1383 RSC; S. S. Babu, V. K. Praveen and A. Ajayaghosh, Chem. Rev., 2014, 114, 1973–2129 CrossRef; J. Raeburn, A. Z. Cardoso and D. J. Adams, Chem. Soc. Rev., 2013, 42, 5143–5156 RSC.
- K. L. Morris, L. Chen, J. Raeburn, O. R. Sellick, P. Cotanda, A. Paul, P. C. Griffiths, S. M. King, R. K. O'Reilly, L. C. Serpell and D. J. Adams, Nat. Commun., 2013, 4, 1480 CrossRef CAS PubMed; D. J. Cornwell, B. O. Okesola and D. K. Smith, Angew. Chem., Int. Ed., 2014, 126, 12669–12673 CrossRef; D. J. Cornwell, O. J. Daubney and D. K. Smith, J. Am. Chem. Soc., 2015, 137, 15486–15492 CrossRef PubMed; Z. Sun, Z. Li, Y. He, R. Shen, L. Deng, M. Yang, Y. Liang and Y. Zhang, J. Am. Chem. Soc., 2013, 135, 13379–13386 CrossRef PubMed.
- T. Yoshii, M. Ikeda and I. Hamachi, Angew. Chem., Int. Ed., 2014, 126, 7392–7395 CrossRef CAS; J. Li, J. Carnall, M. C. Stuart and S. Otto, Angew. Chem., Int. Ed., 2011, 50, 8384–8386 CrossRef PubMed; A. Gopal, M. Hifsudheen, S. Furumi, M. Takeuchi and A. Ajayaghosh, Angew. Chem., Int. Ed., 2012, 124, 10657–10661 CrossRef; M. He, J. Li, S. Tan, R. Wang and Y. Zhang, J. Am. Chem. Soc., 2013, 135, 18718–18721 CrossRef PubMed; B. Xue, Y. Li, F. Yang, C. Zhang, M. Qin, Y. Cao and W. Wang, Nanoscale, 2014, 6, 7832–7837 RSC.
- A. R. Hirst, S. Roy, M. Arora, A. K. Das, N. Hodson, P. Murray, S. Marshall, N. Javid, J. Sefcik, J. Boekhoven, J. H. Van Esch, S. Santabarbara, N. T. Hunt and R. V. Ulijin, Nat. Chem., 2010, 2, 1089–1094 CrossRef CAS PubMed; C. G. Pappas, I. R. Sasselli and R. V. Ulijn, Angew. Chem., Int. Ed., 2015, 127, 8237–8241 CrossRef; S. K. M. Nalluri, C. Berdugo, N. Javid, P. W. Frederix and R. V. Ulijn, Angew. Chem., Int. Ed., 2014, 126, 5992–5997 CrossRef; J. Zhou, X. Du, J. Li, N. Yamagata and B. Xu, J. Am. Chem. Soc., 2015, 137, 10040–10043 CrossRef PubMed; J. Nanda, A. Biswas, B. Adhikari and A. Banerjee, Angew. Chem., Int. Ed., 2013, 52, 5041–5045 CrossRef PubMed; A. K. Das, I. Maity, H. S. Parmar, T. O. McDonald and M. Konda, Biomacromolecules, 2015, 16, 1157–1168 CrossRef PubMed.
- W. Edwards and D. K. Smith, J. Am. Chem. Soc., 2014, 136, 1116–1124 CrossRef CAS PubMed; C. M. Micklitsch, P. J. Knerr, M. C. Branco, R. Nagarkar, D. J. Pochan and J. P. Schneider, Angew. Chem., Int. Ed., 2011, 123, 1615–1617 CrossRef; M.-O. M. Piepenbrock, G. O. Lloyd, N. Clarke and J. W. Steed, Chem. Rev., 2009, 110, 1960–2004 CrossRef PubMed; J. W. Steed, Chem. Soc. Rev., 2010, 39, 3686–3699 RSC; J. S. Foster, J. M. Żurek, N. M. Almeida, W. E. Hendriksen, V. A. le Sage, V. Lakshminarayanan, A. L. Thompson, R. Banerjee, R. Eelkema and H. Mulvana, J. Am. Chem. Soc., 2015, 137, 14236–14239 CrossRef PubMed; Z. Shen, Y. Jiang, T. Wang and M. Liu, J. Am. Chem. Soc., 2015, 137, 16109–16115 CrossRef PubMed; J. Boekhoven, W. E. Hendriksen, G. J. Koper, R. Eelkema and J. H. van Esch, Science, 2015, 349, 1075–1079 CrossRef PubMed.
- C. J. Bowerman and B. L. Nilsson, J. Am. Chem. Soc., 2010, 132, 9526–9527 CrossRef CAS PubMed; C. Ren, Z. Song, W. Zheng, X. Chen, L. Wang, D. Kong and Z. Yang, Chem. Commun., 2011, 47, 1619–1621 RSC; X. Miao, W. Cao, W. Zheng, J. Wang, X. Zhang, J. Gao, C. Yang, D. Kong, H. Xu, L. Wang and Z. Yang, Angew. Chem., Int. Ed., 2013, 52, 7781–7785 CrossRef PubMed; Y. Zhang, B. Zhang, Y. Kuang, Y. Gao, J. Shi, X. X. Zhang and B. Xu, J. Am. Chem. Soc., 2013, 135, 5008–5011 CrossRef PubMed.
- S. Chen, L. Rong, Q. Lei, P. Cao, S. Qin, D. Zheng, H. Jia, J. Zhu, S. Cheng, R. Zhuo and X. Zhang, Biomaterials, 2016, 77, 149–163 CrossRef CAS PubMed; J. Liu, J. Liu, L. Chu, Y. Zhang, H. Xu, D. Kong, Z. Yang, C. Yang and D. Ding, ACS Appl. Mater. Interfaces, 2014, 6, 5558–5565 CrossRef PubMed; S. Qin, M. Peng, L. Rong, B. Li, S. Wang, S. Cheng, R. Zhuo and X. Zhang, Regener. Biomater., 2015, 2, 159–166 CrossRef PubMed; T. Su, Z. Tang, H. He, W. Li, X. Wang, C. Liao, Y. Sun and Q. Wang, Chem. Sci., 2014, 5, 4204–4209 RSC; D. Das, T. Kar and P. K. Das, Soft Matter, 2012, 8, 2348–2365 RSC; Y. Yuan, L. Wang, W. Du, Z. Ding, J. Zhang, T. Han, H. Zhang and G. Ling, Angew. Chem., Int. Ed., 2015, 54, 9700–9704 CrossRef PubMed.
- R. A. Pires, Y. M. Abul-Haija, D. S. Costa, R. Novoa-Carballal, R. L. Reis, R. V. Ulijn and I. Pashkuleva, J. Am. Chem. Soc., 2015, 137, 576–579 CrossRef CAS PubMed; Y. Kuang, J. Shi, J. Li, D. Yuan, K. A. Alberti, Q. Xu and B. Xu, Angew. Chem., Int. Ed., 2014, 53, 8104–8107 CrossRef PubMed; J. Li, Y. Kuang, J. Shi, J. Zhou, J. E. Medina, R. Zhou, D. Yuan, C. Yang, H. Wang, Z. Yang, J. Liu, D. Dinulescu and B. Xu, Angew. Chem., Int. Ed., 2015, 127, 13505–13509 CrossRef; H. Wang, Z. Feng, D. Wu, K. J. Fritzsching, M. Rigney, J. Zhou, Y. Jiang, K. Schmidt-Rohr and B. Xu, J. Am. Chem. Soc., 2016, 138, 10758–10761 CrossRef PubMed; Z. Zheng, P. Chen, M. Xie, C. Wu, Y. Luo, W. Wang, J. Jiang and G. Liang, J. Am. Chem. Soc., 2016, 138, 11128–11131 CrossRef PubMed.
- J. S. Rudra, T. Sun, K. C. Bird, M. D. Daniels, J. Z. Gasiorowski, A. S. Chong and J. H. Collier, ACS Nano, 2012, 6, 1557–1564 CrossRef CAS; J. S. Rudra, S. Mishra, A. S. Chong, R. A. Mitchell, E. H. Nardin, V. Nussenzweig and J. H. Collier, Biomaterials, 2012, 33, 6476–6484 CrossRef; J. Chen, R. R. Pompano, F. W. Santiago, L. Maillat, R. Sciammas, T. Sun, H. Han, D. J. Topham, A. S. Chong and J. H. Collier, Biomaterials, 2013, 34, 8776–8785 CrossRef; G. A. Hudalla, T. Sun, J. Z. Gasiorowski, H. Han, Y. F. Tian, A. S. Chong and J. H. Collier, Nat. Mater., 2014, 13, 829–836 CrossRef PubMed; Y. Tian, H. Wang, Y. Liu, L. Mao, W. Chen, Z. Zhu, W. Liu, W. Zheng, Y. Zhao and D. Kong, Nano Lett., 2014, 14, 1439–1445 CrossRef PubMed; H. Wang, Z. Luo, Y. Wang, T. He, C. Yang, C. Ren, L. Ma, C. Gong, X. Li and Z. Yang, Adv. Funct. Mater., 2016, 26, 1822–1829 CrossRef.
- C. Ren, H. Wang, D. Mao, X. Zhang, Q. Fengzhao, Y. Shi, D. Ding, D. Kong, L. Wang and Z. Yang, Angew. Chem., Int. Ed., 2015, 54, 4823–4827 CrossRef CAS PubMed; C. Ren, J. Zhang, M. Chen and Z. Yang, Chem. Soc. Rev., 2014, 43, 7257–7266 RSC; R. Peltier, G. Chen, H. Lei, M. Zhang, L. Gao, S. S. Lee, Z. Wang and H. Sun, Chem. Commun., 2015, 51, 17273–17276 RSC; Y. Cai, J. Zhan, H. Shen, D. Mao, S. Ji, R. Liu, B. Yang, D. Kong, L. Wang and Z. Yang, Anal. Chem., 2015, 88, 740–745 CrossRef PubMed; T. Xu, C. Liang, S. Ji, D. Ding, D. Kong, L. Wang and Z. Yang, Anal. Chem., 2016, 88, 7318–7323 CrossRef; L. L. Lock, C. D. Reyes, P. Zhang and H. Cui, J. Am. Chem. Soc., 2016, 138, 3533–3540 CrossRef.
- S. G. Rhee, Science, 2006, 312, 1882–1883 CrossRef.
- J. Fang, T. Seki and H. Maeda, Adv. Drug Delivery Rev., 2009, 61, 290–302 CrossRef CAS PubMed; G. Groeger, C. Quiney and T. G. Cotter, Antioxid. Redox Signaling, 2009, 11, 2655–2671 CrossRef.
- B. C. Dickinson and C. J. Chang, J. Am. Chem. Soc., 2008, 130, 9638–9639 CrossRef CAS PubMed.
- J. Kwon, J. Kim, S. Park, G. Khang, P. M. Kang and D. Lee, Biomacromolecules, 2013, 14, 1618–1626 CrossRef CAS.
- E. O. Hileman, J. Liu, M. Albitar, M. J. Keating and P. Huang, Cancer Chemother. Pharmacol., 2004, 53, 209–219 CrossRef CAS PubMed; S. Kawanishi, Y. Hiraku, S. Pinlaor and N. Ma, J. Biol. Chem., 2006, 387, 365–372 Search PubMed.
- K. Sugamura and J. F. Keaney, Free Radical Biol. Med., 2011, 51, 978–992 CrossRef CAS.
- K. J. Barnham, C. L. Masters and A. I. Bush, Nat. Rev. Drug Discovery, 2004, 3, 205–214 CrossRef CAS.
- D. Lee, S. Khaja, J. C. Velasquez-Castano, M. Dasari, C. Sun, J. Petros, W. R. Taylor and N. Murthy, Nat. Mater., 2007, 6, 765–769 CrossRef CAS.
- M. Ikeda, T. Tanida, T. Yoshii, K. Kurotani, S. Onogi, K. Urayama and I. Hamachi, Nat. Chem., 2014, 6, 511–518 CrossRef CAS; T. Yoshii, S. Onogi, H. Shigemitsu and I. Hamachi, J. Am. Chem. Soc., 2015, 137, 3360–3365 CrossRef.
- C. Perez, J.-P. Monserrat, Y. Chen and S. M. Cohen, Chem. Commun., 2015, 51, 7116–7119 RSC.
- J. Raeburn, G. Pont, L. Chen, Y. Cesbron, R. Lévy and D. J. Adams, Soft Matter, 2012, 8, 1168–1174 RSC.
- R. Kumar, J. Han, H. J. Lim, W. Ren, J. Y. Lim, J. H. Kim and J. S. Kim, J. Am. Chem. Soc., 2014, 136, 17836–17843 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization, hydrogel formation, determination of conversion percentage, rheological data. See DOI: 10.1039/c6ra26536g |
| This journal is © The Royal Society of Chemistry 2017 |