Curcumin loaded mesoporous silica: an effective drug delivery system for cancer treatment†
Rajesh
Kotcherlakota
a,
Ayan Kumar
Barui
ab,
Sanjiv
Prashar
c,
Mariano
Fajardo
c,
David
Briones
d,
Antonio
Rodríguez-Diéguez
d,
Chitta Ranjan
Patra
*ab and
Santiago
Gómez-Ruiz
*c
aBiomaterials Group, CSIR-Indian Institute of Chemical Technology, Uppal Road, Tarnaka, Hyderabad 500007, India. E-mail: crpatra@iict.res.in; patra.chitta@gmail.com
bAcademy of Scientific and Innovative Research (AcSIR), Training and Development Complex, CSIR Campus, CSIR Road, Taramani, Chennai – 600 113, India
cDepartamento de Biología y Geología, Física y Quimica Inorgánica, E.S.C.E.T., Universidad Rey Juan Carlos, Calle Tulipán s/n, 28933, Móstoles, Madrid, Spain. E-mail: santiago.gomez@urjc.es
dDepartamento de Química Inorgánica, Universidad de Granada, Facultad de Ciencias, Fuente Nueva s/n 18071, Granada, Spain
First published on 16th December 2015
Abstract
In the present study, we report the delivery of anti-cancer drug curcumin to cancer cells using mesoporous silica materials. A series of mesoporous silica material based drug delivery systems (S2, S4 and S6) were first designed and developed through the amine functionalization of KIT-6, MSU-2 and MCM-41 followed by the loading of curcumin. The curcumin loaded materials were characterized with several physico-chemical techniques and thoroughly screened on cancer cells to evaluate their in vitro drug delivery efficacy. All the curcumin loaded silica materials exhibited higher cellular uptake and inhibition of cancer cell viability compared to pristine curcumin. The effective internalization of curcumin in cancer cells through the mesoporous silica materials initiated the generation of intracellular reactive oxygen species and the down regulation of poly ADP ribose polymerase (PARP) enzyme levels compared to free curcumin leading to the activation of apoptosis. This study shows that the anti-cancer activity of curcumin can be potentiated by loading onto mesoporous silica materials. Therefore, we strongly believe that mesoporous silica based curcumin loaded drug delivery systems may have future potential applications for the treatment of cancers.
Introduction
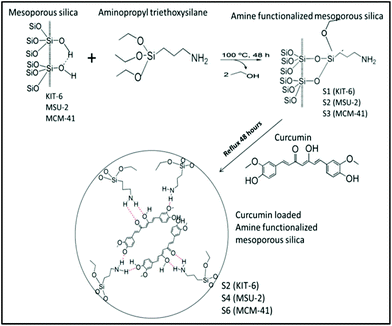
Curcuma longa has extensively been used as a food additive and conservant in India, China and other Asian countries.1 In addition, it has been used for domestic remediation in Chinese medicine for different diseases such as diabetes, hepatic dysfunctions and other health problems.2 For this reason, during the last decades a high number of studies have been carried out in order to determine the biological activity and pharmacological properties of Curcuma longa and its extracts.3 Curcumin is the major curcuminoid and the main bioactive component of Curcuma longa. It has diverse biological applications as an antioxidant, antimutagenic, antidiabetic, antibacterial, antifungal and especially as an anticancer agent.4 Curcumin has also been used in clinical trials for the reduction of the inflammatory processes after surgery.5 Furthermore, earlier reports demonstrate that curcumin is cytotoxic to various cancer cells through the induction of apoptosis and decrease of cell invasiveness of the tumoural area.6 Dose dependent toxicity studies in normal cell lines suggest that curcumin is well tolerated at high doses without any toxic effect. However, the administration of curcumin in the human body as an anti-cancer agent has not been found to be effective due to its lower systemic bioavailability originating from its low solubility and instability.7 To overcome these limitations, researchers have been engaged in making different formulations such as the encapsulation of curcumin with polymeric nanoparticles or silicalization of curcumin-loaded solid lipid nanoparticle (SLN)/micelle dispersions,8 metal or non-metal nanoparticles, phospholipids, microemulsions or by the preparation of other curcumin analogues.9 Amongst all these approaches, the prevalent one is the encapsulation of curcumin with nanoparticles. However, rigorous studies are still needed to evaluate the efficacy and toxicity of the nanoparticles.10a,bA different approach for developing novel drug-delivery systems is the use of mesoporous silica materials because of their interesting properties, such as (i) variable and controllable particle sizes ranging from 50 nm to microns that lead to an easy endocytosis by cells and possess low cytotoxicity, (ii) stability to heat, pH, mechanical stress and chemical degradations, (iii) tunable narrow pore size distribution and pore diameter that lead to a rational loading of different drug molecules, (iv) high surface area which allows a good degree of drug incorporation and (v) easy functionalization of the external and internal surface by different ligands, metal complexes, biomaterials or other nanostructures.10b For all these reasons, silica-based mesoporous materials have been used as drug delivery vehicles in various biomedical applications, such as anti-inflammatory, analgesic, bone regeneration and also in anti-cancer treatments.10c–e
Considering their biomedical applications, the mesoporous silica materials KIT-6, MSU-2 and MCM-41 have been chosen as models with high porosity and capacity of loading of curcumin in order to study their biological behaviour as drug-delivery systems, as a first step before a plausible therapeutic application. In this context, we have synthesized a series of silica based mesoporous materials (KIT-6, MSU-2 and MCM-41, with different particle sizes, pore sizes and morphology), which have been functionalized with 3-aminopropyltriethoxysilane (APTES) to give rise to amine functionalized materials (S1, S3 and S5). Further, we have developed S1, S3 and S5 based drug delivery systems by conjugating them with curcumin, namely S2, S4 and S6, respectively. All the materials have been characterized by several physico-chemical techniques such as TEM (transmission electron microscopy), SEM (scanning electron microscopy), FT-IR (Fourier transform infrared spectroscopy), TGA (thermogravimetric analysis) and BET (Brunauer–Emmett–Teller) analysis. Release kinetic studies with the curcumin loaded mesoporous materials (S2, S4 and S6) reveal a slow and sustained release of curcumin under physiological conditions (pH: 7.4). The in vitro cell culture study of the synthesized materials shows that all of them are biocompatible for normal cell lines (NIH-3T3 and CHO). However, the curcumin loaded mesoporous silica materials, especially S4, are found to be cytotoxic towards different cancer cells (A549, MCF-7 and SKOV3) compared to free curcumin. The generation of intracellular ROS (O2˙−) and the down regulation of PARP expression levels leading to the activation of apoptosis have been found to be the molecular mechanisms behind the anti-cancer potential of curcumin loaded mesoporous silica materials. In addition, although some studies using APTES as an immobilizing agent have been previously reported, no materials have been published to date with the combination of APTES–curcumin and all the spectroscopic and biological data of the final materials S2, S4 and S6 are new. These results show the future potential applications of mesoporous silica-based materials as drug delivery systems for the treatment of several tumours.
Experimental section
Chemistry
Preparation of template materials
Preparation of amine functionalized materials
Preparation of curcumin loaded materials
Release studies in biological conditions
The curcumin release from the materials in biological conditions was carried out in a blood simulated fluid (tris buffer: pH 7.4).14a 5 mL of the mixture of aqueous buffer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol (95
ethanol (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5; v/v) was added to 10 mg of the studied materials. These suspensions were incubated at 37 °C in a water bath for different time periods up to 240 hours. The suspension was then centrifuged and filtered through a nylon filter (0.45 μm) and the filtrate was diluted to the working concentration with ethanol. The quantification of the curcumin was immediately carried out measuring the absorbance of the solutions using UV-Visible spectroscopy at λmax of 428 nm.
5; v/v) was added to 10 mg of the studied materials. These suspensions were incubated at 37 °C in a water bath for different time periods up to 240 hours. The suspension was then centrifuged and filtered through a nylon filter (0.45 μm) and the filtrate was diluted to the working concentration with ethanol. The quantification of the curcumin was immediately carried out measuring the absorbance of the solutions using UV-Visible spectroscopy at λmax of 428 nm.
Biology
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well were seeded into 96 well plates and incubated for 24 hours. Then the cells were treated with different materials in a dose dependent manner (considering the loading of curcumin on the material) for a further 48 hours. All the materials were irradiated under UV light prior to treatment. After 48 hours, 100 µL of MTT reagent (0.5 mg mL−1) was added to each well of the plate by replacing the old media and incubated in the dark for 4 hours. The in situ formed formazan dye was then solubilized by DMSO–methanol (1
000 cells per well were seeded into 96 well plates and incubated for 24 hours. Then the cells were treated with different materials in a dose dependent manner (considering the loading of curcumin on the material) for a further 48 hours. All the materials were irradiated under UV light prior to treatment. After 48 hours, 100 µL of MTT reagent (0.5 mg mL−1) was added to each well of the plate by replacing the old media and incubated in the dark for 4 hours. The in situ formed formazan dye was then solubilized by DMSO–methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; v/v) solvent mixture and kept on the shaker for homogeneous mixing for a few minutes. The absorbance of the purple colour dye developed was measured in a micro plate reader (ELx 800 MS) at 570 nm. All the experiments were carried out in triplicate and the results were expressed as normalized viability.
1; v/v) solvent mixture and kept on the shaker for homogeneous mixing for a few minutes. The absorbance of the purple colour dye developed was measured in a micro plate reader (ELx 800 MS) at 570 nm. All the experiments were carried out in triplicate and the results were expressed as normalized viability.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 minutes at 4 °C and the supernatant obtained was used for the immunoblot procedures. Protein estimation was carried out using the Bradford assay method and equal concentrations of protein samples were loaded in 8% denaturing sodium dodecyl sulfate poly acrylamide gel. After electrophoretic separation of the proteins, they were blotted on the nitrocellulose membrane and blocked in 5% BSA solution for 2 hours. The membrane was incubated with anti-PARP monoclonal primary antibody and anti GAPDH primary antibody in a TBST (Tween-20 (TBS-T; 10 mM Tris, pH 7.5, 150 mM NaCl, 0.05% Tween-20)) solution (dilutions were followed according to manufactures instructions) for 2 hours at room temperature. The membrane was then washed in the TBST solution twice and incubated with the secondary antibody (conjugated with alkaline phosphatase) for 1 hour at room temperature. The blot was developed using the BCIP–NBT solution in the dark.
000 rpm for 10 minutes at 4 °C and the supernatant obtained was used for the immunoblot procedures. Protein estimation was carried out using the Bradford assay method and equal concentrations of protein samples were loaded in 8% denaturing sodium dodecyl sulfate poly acrylamide gel. After electrophoretic separation of the proteins, they were blotted on the nitrocellulose membrane and blocked in 5% BSA solution for 2 hours. The membrane was incubated with anti-PARP monoclonal primary antibody and anti GAPDH primary antibody in a TBST (Tween-20 (TBS-T; 10 mM Tris, pH 7.5, 150 mM NaCl, 0.05% Tween-20)) solution (dilutions were followed according to manufactures instructions) for 2 hours at room temperature. The membrane was then washed in the TBST solution twice and incubated with the secondary antibody (conjugated with alkaline phosphatase) for 1 hour at room temperature. The blot was developed using the BCIP–NBT solution in the dark.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well) and incubated with S4 (10 µM with respect to curcumin) and curcumin (10 µM) for 24 hours. The cells were then washed and fixed in a 4% formaldehyde solution for 5 minutes and permeabilised with 0.05% triton-X for 10 minutes. A Hoechst-33258–PI staining solution (containing RNase) was added to the cells and incubated for 30 minutes in the dark. Cells were then washed 3 times with DPBS and observed by fluorescence microscopy. The red fluorescence (λEm = 610 nm) images were collected using a Nikon fluorescence microscope after excitation at λEx = 535 nm. The blue fluorescent images (λEm = 485 nm) of the Hoechst stained nuclei were obtained after excitation at (λEx) at 380 nm.
000 cells per well) and incubated with S4 (10 µM with respect to curcumin) and curcumin (10 µM) for 24 hours. The cells were then washed and fixed in a 4% formaldehyde solution for 5 minutes and permeabilised with 0.05% triton-X for 10 minutes. A Hoechst-33258–PI staining solution (containing RNase) was added to the cells and incubated for 30 minutes in the dark. Cells were then washed 3 times with DPBS and observed by fluorescence microscopy. The red fluorescence (λEm = 610 nm) images were collected using a Nikon fluorescence microscope after excitation at λEx = 535 nm. The blue fluorescent images (λEm = 485 nm) of the Hoechst stained nuclei were obtained after excitation at (λEx) at 380 nm.
Results and discussion
Chemistry
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 and MCM-41
12 and MCM-41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 mesoporous silicas as the host materials, according to earlier reports.
13 mesoporous silicas as the host materials, according to earlier reports.
Porous materials can usually be impregnated simply in a concentrated solution of the cargo even without functionalization. However, direct impregnation of curcumin on the host materials KIT-6, MSU-2 and MCM-41 under different reaction conditions does not yield high loading contents of curcumin on the materials.
According to previous studies14a of the betulinic acid loaded MCM-41 system, the functionalization of the material MCM-41 with amine groups increased the loading content of betulinic acid by the formation of weak intermolecular forces between the amine group of the functionalized materials and the polar groups of betulinic acid.
Additionally, amine functionalized materials have previously been synthesized for a wide range of applications.15 Following the reported protocols,14a the grafting reactions of 3-aminopropyltriethoxysilane (APTES: AP) with the host materials KIT-6, MSU-2 and MCM-41 were carried out to give rise to the amine functionalized materials S1 (KIT-6-AP), S3 (MSU-2-AP) and S5 (MCM-41-AP), respectively (Scheme 1).
| Materials | Description of materials | S BET (m2 g−1) | V p (cm3 g−1) | d p (Å) |
|---|---|---|---|---|
| KIT-6 | Mesoporous silica-1 | 700 | 0.60 | 76.2 |
| S1 | Functionalized-KIT-6 (KIT-6-AP) | 130 | 0.20 | 62.5 |
| S2 | Curcumin loaded-S1 (KIT-6-AP-CUR) | 20 | 0.04 | <18 |
| MSU-2 | Mesoporous silica-2 | 843 | 0.97 | 51.1 |
| S3 | Functionalized-MSU-2 (MSU-2-AP) | 470 | 0.67 | 39.5 |
| S4 | Curcumin loaded-S3 (MSU-2-AP-CUR) | 425 | 0.49 | 35.4 |
| MCM-41 | Mesoporous silica-3 | 1117 | 1.12 | 29.6 |
| S5 | Functionalized-MCM-41 (MCM-41-AP) | 650 | 0.25 | 18.3 |
| S6 | Curcumin loaded-S5 (MCM-41-AP-CUR) | 414 | 0.17 | <18 |
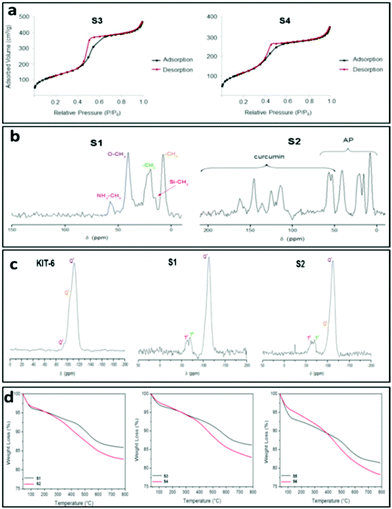
It is important to mention that the isotherm of material S1 (Fig. S1†) changes to a type III isotherm after loading with curcumin (S2) with a very thin hysteresis loop which is indicative of a microporous or non-porous material, suggesting that physisorption of curcumin may completely block the pores of the system.11a It is noteworthy that hysteresis loop in materials S1 and S6 was not closed. However, for both materials, the desorption curve is very close to that of the adsorption at very low relative pressures. The very low difference in the desorption step is not significant to be considered as a different behaviour in nitrogen desorption. In addition, after functionalization either with APTS or with curcumin all the materials showed a decrease of the surface area which is higher in ratio in the case of KIT-6-based materials, as is expected due to the higher number of silanol groups in their structure.11b
The unmodified KIT-6, MSU-2 and MCM-41 displayed X-ray diffractions (XRD) which were typical for the mesoscopic order of mesoporous materials (Table 2). The result showed a well-resolved pattern at low 2θ with a very sharp diffraction peak for KIT-6, MSU-2 and MCM-41 at 0.9, 1.4 and 2.2°, respectively. These characteristic Bragg's peaks correspond to reflections from the (100) plane in MSU-2 and MCM-41 and the (211) plane in KIT-6. In addition, the MCM-41 diffractogram showed a weak peak at 3.9° which corresponds to reflections from the (110) planes.
| Material | hkl | 2θ (°) | d (100) (Å) | a 0 (Å) | Size (nm) | Charge (mV) |
|---|---|---|---|---|---|---|
| KIT-6 | 211 | 0.9 | 97.3 | 112.4 | — | — |
| S1 | 211 | 0.9 | 97.0 | 112.0 | 5577 | −9.36 |
| S2 | 211 | 0.9 | 96.7 | 111.8 | 1983 | −18.3 |
| MSU-2 | 100 | 1.4 | 62.1 | 71.7 | — | — |
| S3 | 100 | 1.3 | 68.1 | 78.6 | 3781 | −0.799 |
| S4 | 100 | 1.3 | 66.2 | 76.5 | 1915 | 7.16 |
| MCM-41 | 100 | 2.2 | 39.5 | 45.6 | — | — |
| S5 | 100 | 2.4 | 37.2 | 42.9 | 2124 | 12.3 |
| S6 | 100 | 2.3 | 37.9 | 43.9 | 1233 | −23.2 |
A significant decrease in the intensity of the diffraction peaks was also observed after the loading of curcumin compared to the amine functionalized materials. This is due to the blocking of the dispersion centres by the organic fragment (AP and/or curcumin) for diffractograms of mesoporous silica materials (Fig. S3–S6†).
The incorporation of the 3-aminopropyl group was also confirmed by multinuclear MAS NMR spectroscopy. The 13C-CP MAS NMR spectrum of S1 (Fig. 1c) showed a set of five signals between ca. 10 and 60 ppm corresponding to the three carbon atoms of the methylene groups of the alkyl chain of AP along with two intense signals corresponding to the carbon atoms of the ethoxy moieties. On the other hand, the 13C-CP MAS NMR spectrum of the curcumin loaded material S2 (Fig. 1c) showed the appearance of two new broad signals of low intensity at around 50 ppm, corresponding to the methoxy carbon atoms of the curcumin and a set of new signals between 100 and 200 ppm assigned to the sp2 carbon atoms (aromatic and carbonyl) of the molecule. Similar results could be anticipated for the other set of materials (S3–S4; S5–S6).
The amine-functionalization of silica materials was confirmed by 29Si MAS NMR spectroscopy. The mesoporous silica materials exhibit typical Q2, Q3 and Q4 peaks in the 29Si MAS NMR spectra17 as observed in the case of host material KIT-6 (Fig. 1b).
On the other hand, the 29Si MAS NMR spectrum of S1 (Fig. 1b) showed a substantial decrease in the intensity of Q2 and Q3 sites in comparison to that of the host material KIT-6. The spectra of S1 also exhibited the appearance of two additional peaks of low intensity at ca. −55 and −66 ppm corresponding to the T2 ((SiO)2SiOH-R) and T3 ((SiO)3Si-R) sites, respectively, which come from the functionalization with the 3-aminopropyltriethoxysilane. Further, the curcumin loaded material S2 (Fig. 1b) showed slight differences in their 29Si MAS NMR spectra compared to the spectrum of S1 (Fig. 1b). Similar results could be expected for the other set of materials (MSU-2, S3 and S4; MCM-41, S5 and S6, see Fig. S7 and S8†). The results altogether suggest an amine functionalization in the corresponding host materials.
In the FT-IR spectra of S2, S4 and S6 some typical bands corresponding to the curcumin loading were observed with special importance at ca. 1520 cm−1, which can be attributed to the carbonyl groups of curcumin (Fig. S9–S11†).
The quantity of organic groups attached to the material was determined by TGA and elemental analysis leading to an amine functionalization of 9.1, 8.5 and 11.0 wt% for the materials S1, S3 and S5, respectively (Table 3). Furthermore, in order to determine the N%, an elemental analysis was carried out on the materials S1, S3 and S5 (Table S1,†Fig. 1d and S12†). Analyzing the elemental analyses data, it was found that the experimental and calculated N% are very similar to the functionalization rates (in mmol of compound per gram of material and in wt%). However, the C% showed that the functionalization reaction of APTES occurred through the elimination of only two ethanol molecules while one ethoxy group was retained on the supported amino ligand (Scheme 1). The TGA and elemental analysis results also showed that the loading contents of curcumin in S2, S4 and S6 materials were ca. 2.7, 3.6 and 3.6%, respectively (Table 3, Fig. 1d and S12†). Thus, the encapsulation efficiency of each of the studied materials S2, S4 and S6 were found to be 13.4, 17.8 and 17.9%, respectively.
| Material | % weight loss (20–100 °C) | % weight loss (100–600 °C) | % curcumin |
|---|---|---|---|
| S1 | 3.62 | 9.08 | — |
| S2 | 3.28 | 11.76 | 2.68 |
| S3 | 2.93 | 8.46 | — |
| S4 | 2.32 | 14.34 | 3.56 |
| S5 | 5.31 | 11.00 | — |
| S6 | 3.75 | 14.58 | 3.58 |
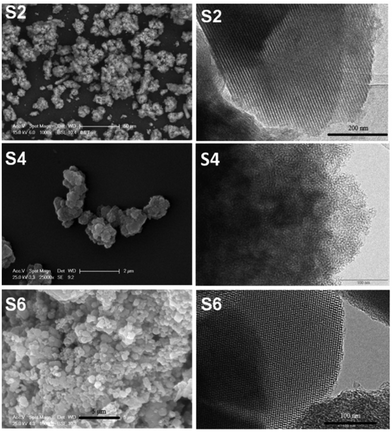
Finally, the mesoporous silica based materials were characterized by SEM and TEM. The SEM image of KIT-6 showed that this material (solid state) does not a well-defined particle shape and forms aggregates of microns (5.4 ± 2.2 µm) while the SEM image of MCM-41 showed that it consists of hexagonal or quasi-spherical particles of size ca. 700 nm (708 ± 157 nm) (Fig. S13†). On the other hand, the particle size of MSU-2 is found to be 439 ± 97 nm, as observed from the SEM images (calculated using Image J analyzing software) of the material (Fig. S13†).
The TEM images of KIT-6 and MCM-41 show the ordered arrangements of the pores while in the case of MSU-2 a disordered arrangement of the pores was observed (Fig. S14†). The curcumin loaded materials S2, S4 and S6 were also characterized by SEM and TEM, which showed that they maintained their original morphology, particle size and arrangement, and pore distribution (Fig. 2) with respect to the host materials KIT-6, MSU-2 and MCM-41. Additionally, a dynamic light scattering (DLS) study was carried out with all S1–S6 materials and the results are shown in Table 2 and in Fig. S15–S20.†
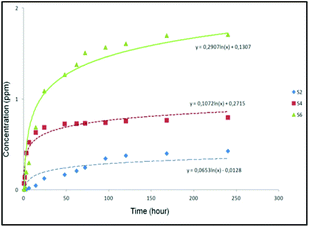
The results suggested that the release of curcumin was higher in the case of S6 compared to that of S2 and S4 (Fig. 3). It was observed that S6 released 1.8 ppm curcumin at pH 7.4 (corresponding to 2.3 wt% loaded curcumin) after 240 hours. On the other hand, materials S2 and S4 released less quantity of curcumin (0.4 and 0.8 ppm corresponding to 0.8 and 1.1 wt% of the loaded curcumin, respectively) into the studied medium after 240 hours. In a direct comparison between materials S6 with the other curcumin loaded materials (S2 and S4), S6 seems to present a slightly faster release which might be a consequence of the higher loading of curcumin in the external surface area of S6 compared to that of S2 and S4.
Biology
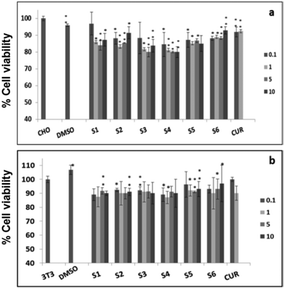
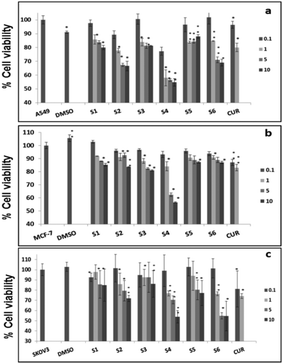
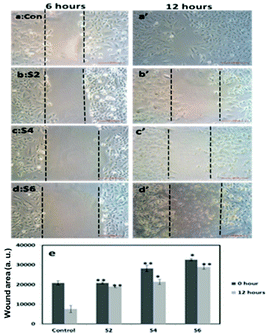
Further, cell viability assay was also carried out in different cancer cell lines, such as A549, MCF-7 and SKOV3. The results showed that all the curcumin loaded materials (S2, S4 and S6) were cytotoxic in A549 (Fig. 5a) and SKOV3 (Fig. 5b) cells compared to the free curcumin. However, only the S4 material showed significant cytotoxicity in MCF-7 cells (Fig. 5c), which may be due to the enhanced uptake of S4 in cancer cells compared to other curcumin loaded materials (S2 and S6). The amine functionalized mesoporous silica materials (S1, S3 and S5) also exhibited a slight cytotoxicity in the respective cancer cell lines, which may be due to the functionalization effect which corroborates the earlier reports.20
It has previously been reported that curcumin demonstrates a dual nature to be both a neuroregenerative agent in endogenous neural stem cells (NSC)21 as well as a cytotoxic agent in cancer cells,22 demonstrating its versatile properties in different cell types. Similarly, in the present study, the results indicated that the curcumin present in the mesoporous materials (S2, S4 and S6) were biocompatible in normal cells and cytotoxic in cancer cells. Our results have further been evidenced by an earlier study23 that showed a greater curcumin uptake in cancer cells compared to normal NIH 3T3 cells, which may be the reason for the biocompatibility of curcumin present in mesoporous materials in normal cells and its cytotoxicity in cancer cells. These results altogether suggest that the curcumin loaded materials, especially S4, enhance the bioavailability of curcumin by partially overcoming the problems associated with the low systematic bioavailability of curcumin originating from its low solubility and instability, indicating the future potential application of mesoporous silica materials as curcumin drug delivery vehicles in cancer treatments.24
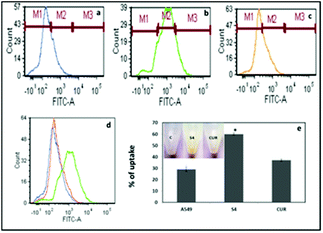
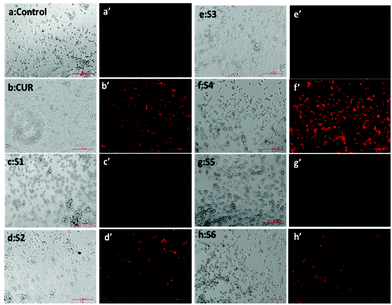
The quantification results also showed that the cellular uptake of curcumin present in S4 (60%) was greater compared to free curcumin (37%) at a similar dose (Fig. 7e). The flow cytometry results also corroborate with the fluorescence microscopy data for cellular internalization of S4.
Interestingly, the maximum cellular uptake was observed for S4 materials which may be evidence of its more cytotoxic nature in different cancer cells. The exact mechanism of cellular uptake of the materials is quite unclear and a thorough investigation is still required, which is beyond the scope of the present study. However it can be assumed that the microsized mesoporous silica materials (>1 µm) may internalize through macro pinocytosis as reported in an earlier study.25
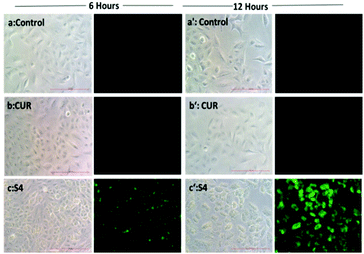
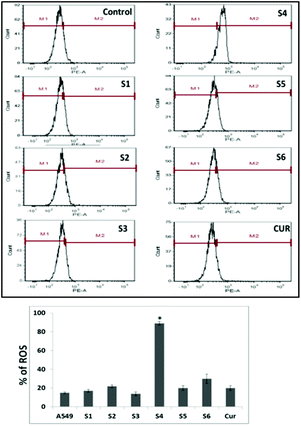
However, S4 treated cells exhibited a significant intense red fluorescence suggesting the excessive formation of the superoxide ion radical. The results corroborate with the internalization and cell viability data revealing the higher uptake of S4 which produced excessive amount of ROS leading to enhanced cytotoxicity in cancer cells.
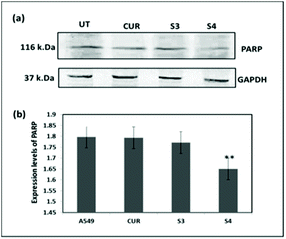
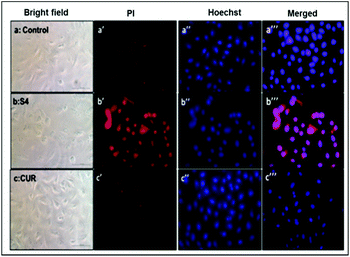
Hence, Hoechst-33258–PI staining was performed according to earlier literature30 in order to analyze the apoptosis induced by the curcumin loaded material S4. The results showed that the untreated cells and cells treated with free curcumin exhibited poor red fluorescence. However the cells treated with S4 showed a brighter red fluorescence, indicating major DNA damage in A549 cells which leads to apoptosis (Fig. 12). The results also correlate with the western blot analysis data which suggests that the down regulation of PARP causes the DNA damage.
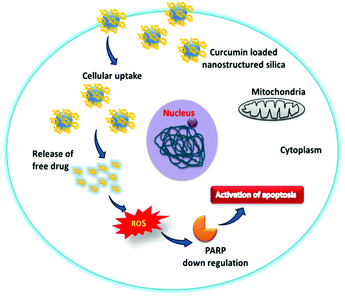
The overall cytotoxic mechanism of curcumin in the mesoporous silica material is presented in Scheme 2. The curcumin loaded mesoporous materials enhance the uptake of curcumin in cancer cells. The generation of the intracellular superoxide ion radical by the internalized curcumin present in the silica materials causes the oxidative stress in cancer cells which subsequently leads to PARP down regulation and DNA damage. The overall mechanism suggests that cancer cell death is through the activation of apoptosis.
Conclusions
We have prepared a series of curcumin loaded mesoporous silica materials which have been thoroughly characterized by different physico-chemical methods. Release kinetic studies with the materials reveal the slow and sustained release of curcumin at physiological pH (7.4). Furthermore, in vitro studies demonstrate that all the curcumin loaded materials, especially S4, show the inhibition of cancer cell viability and migration compared to free curcumin, suggesting the efficacy of the drug delivery system. The intracellular production of reactive oxygen species (ROS), especially O2˙−, and the down regulation of PARP are the molecular mechanisms behind the anti-cancer activity of the curcumin loaded materials. This study demonstrates the future potential application of curcumin loaded silica based mesoporous materials for the treatment of cancer diseases.Acknowledgements
We gratefully acknowledge financial support from the Ministerio de Economía y Competitividad, Spain (Grant no. CTQ-2012-30762). C. Corpa, S. García, E. Hernández and D. Pérez are gratefully acknowledged for helpful discussions. CRP is grateful to DST, New Delhi for ‘Ramanujan Fellowship grant’ (SR/S2/RJN-04/2010; GAP0305) and CSIR, New Delhi for generous financial support from 12th Five Year Plan (FYP) projects (CSC0302 and CSC0111) for this research. AKB is thankful to UGC, New Delhi for senior research fellowship. We are thankful to analytical chemistry division (CSIR-IICT) for helping in the detection of metal content in biological samples by ICPOES technique.References
- H. Zhou, C. S. Beevers and S. Huang, Curr. Drug Targets, 2011, 12, 332 CrossRef CAS PubMed.
- E. J. Seo, V. Kuete, O. Kadioglu, B. Krusche, S. Schroder, H. J. Greten, J. Arend, I. S. Lee and T. Efferth, eCAM, 2013, 131306 Search PubMed.
- R. K. Maheshwari, A. K. Singh, J. Gaddipati and R. C. Srimal, Life Sci., 2006, 78, 2081 CrossRef CAS PubMed.
- Y. Son, J. H. Lee, Y. K. Cheong, H. T. Chung and H. O. Pae, BioMed Res. Int., 2013, 918039 Search PubMed.
- H. Hatcher, R. Planalp, J. Cho, F. M. Torti and S. V. Torti, Cell. Mol. Life Sci., 2008, 65, 1631 CrossRef CAS PubMed.
- (a) G. Bar-Sela, R. Epelbaum and M. Schaffer, Curr. Med. Chem., 2010, 17, 190 CrossRef CAS PubMed; (b) T. Tanaka, J. Carcinog., 2009, 8, 5 CrossRef PubMed; (c) D. Bandyopadhyay, Front. Chem., 2014, 2, 113 Search PubMed; (d) J. L. Yang and W. Zhang, Curr. Opin. Oncol., 2013, 25, 398 CrossRef CAS PubMed; (e) S. S. Dhule, P. Penfornis, T. Frazier, R. Walker, J. Feldman, G. Tan, J. B. He, A. Alb, V. John and R. Pochampally, Nanomed.: Nanotechnol., Biol. Med., 2012, 8, 440 CrossRef CAS PubMed; (f) A. Luetke, P. A. Meyers, I. Lewis and H. Juergens, Cancer Treat. Rev., 2014, 40, 523 CrossRef PubMed.
- V. S. Gota, G. B. Maru, T. G. Soni, T. R. Gandhi, N. Kochar and M. G. Agarwal, J. Agric. Food Chem., 2010, 58, 2095 CrossRef CAS PubMed.
- (a) L. Mamani, S. Nikzad, H. Kheiri-Manjili, S. Al-Musawi, M. Saeedi, S. Askarlou, A. Foroumadi and A. Shafiee, Eur. J. Med. Chem., 2014, 83, 646 CrossRef CAS PubMed; (b) S. Kim, M.-J. Stebe, J.-L. Blin and A. Pasc, J. Mater. Chem. B, 2014, 2, 7910 RSC.
- N. Rocks, S. Bekaert, I. Coia, G. Paulissen, M. Gueders, B. Evrard, J. C. Van Heugen, P. Chiap, J. M. Foidart, A. Noel and D. Cataldo, Br. J. Cancer, 2012, 107, 1083 CrossRef CAS PubMed.
- (a) Y. M. Tsai, W. L. Chang-Liao, C. F. Chien, L. C. Lin and T. H. Tsai, Int. J. Nanomed., 2012, 7, 2957 CrossRef CAS PubMed; (b) O. Naksuriya, S. Okonogi, R. M. Schiffelers and W. E. Hennink, Biomaterials, 2014, 35, 3365 CrossRef CAS PubMed; (c) A. Baeza, M. Colilla and M. Vallet-Regí, Expert Opin. Drug Delivery, 2015, 12, 319 CrossRef CAS PubMed; (d) Y. Wang, Q. Zhao, N. Han, L. Bai, J. Li, J. Liu, E. Che, L. Hu, Q. Zhang, T. Jiang and S. Wang, Nanomed.: Nanotechnol., Biol. Med., 2015, 11, 313 CrossRef CAS PubMed; (e) J. Safari and Z. Zarnegar, J. Saudi Chem. Soc., 2014, 18, 85 CrossRef CAS.
- (a) P. J. Lebed, K. de Souza, F. Bilodeau, D. Lariviere and F. Kleitz, Chem. Commun., 2011, 47, 11525 RSC; (b) F. Kleitz, S. Hei Choi and R. Ryoo, Chem. Commun., 2003, 2136–2137 RSC.
- D. Perez-Quintanilla, A. Sanchez, I. del Hierro, M. Fajardo and I. Sierra, J. Nanosci. Nanotechnol., 2009, 9, 4901 CrossRef CAS PubMed.
- M. V. Landau, S. P. Varkey, M. Herskowitz, O. Regev, S. Pevzner, T. Sen and Z. Luz, Microporous Mesoporous Mater., 1999, 33, 149 CrossRef CAS.
- (a) S. Sanchez-Munoz, S. Gomez-Ruiz, D. Perez-Quintanilla, S. Morante-Zarcero, I. Sierra, S. Prashar, R. Paschke and G. N. Kaluderovic, ChemMedChem, 2012, 7, 670 CrossRef CAS PubMed; (b) S. Mukherjee, D. Chowdhury, R. Kotcherlakota, S. Patra, B. Vinothkumar, M. P. Bhadra, B. Sreedhar and C. R. Patra, Theranostics, 2014, 4, 316 CrossRef PubMed.
- M. Vallet-Regi, F. Balas and D. Arcos, Angew. Chem., Int. Ed., 2007, 46, 7548 CrossRef CAS PubMed.
- K. S. W. Sing, D. H. Everett, R. Haul, L. Moscou, R. A. Pierotti, J. Rouquerol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603 CrossRef CAS.
- G. Engelhardt and D. Michel, High Resolution Solid-State NMR of Silicate and Zeolites, Wiley-Blackwell, 1987 Search PubMed.
- S. Mukherje, P. Sriram, A. K. Barui, S. K. Nethi, V. Veeriah, S. Chatterjee, K. I. Suresh and C. R. Patra, Adv. Healthcare Mater., 2015, 4, 1722 CrossRef PubMed.
- (a) J. Ceballos-Torres, S. Prashar, M. Fajardo, A. Chicca, J. Gertsch, A. B. Pinar and S. Gómez-Ruiz, Organometallics, 2015, 34, 2522 CrossRef CAS; (b) G. N. Kaluđerović, D. Pérez-Quintanilla, I. Sierra, S. Prashar, I. del Hierro, Ž. Žižak, Z. D. Juranić, M. Fajardo and S. Gómez-Ruiz, J. Mater. Chem., 2010, 20, 806 RSC; (c) A. Balbín, F. Gaballo, J. Ceballos-Torres, S. Prashar, M. Fajardo, G. N. Kaluderovic and S. Gómez-Ruiz, RSC Adv., 2014, 4, 54775 RSC.
- (a) A. J. Di Pasqua, K. K. Sharma, Y.-L. Shi, B. B. Toms, W. Ouellette, J. C. Dabrowiak and T. Asefa, J. Inorg. Biochem., 2008, 102, 1416 CrossRef CAS PubMed; (b) Z. Tao, B. B. Toms, J. Goodisman and T. Asefa, Chem. Res. Toxicol., 2009, 22, 1869 CrossRef CAS PubMed; (c) Y. Sanchez, G. P. Simon, E. Calvino, E. de Blas and P. Aller, J. Pharmacol. Exp. Ther., 2010, 335, 114 CrossRef CAS PubMed.
- S. K. Tiwari, S. Agarwal, B. Seth, A. Yadav, S. Nair, P. Bhatnagar, M. Karmakar, M. Kumari, L. K. Chauhan, D. K. Patel, V. Srivastava, D. Singh, S. K. Gupta, A. Tripathi, R. K. Chaturvedi and K. C. Gupta, ACS Nano, 2014, 8, 76 CrossRef CAS PubMed.
- M. A. Khan, S. Gahlot and S. Majumdar, Mol. Cancer Ther., 2012, 11, 1873 CrossRef CAS PubMed.
- K. K. Gupta, S. S. Bharne, K. Rathinasamy, N. R. Naik and D. Panda, FEBS J., 2006, 273, 5320 CrossRef CAS PubMed.
- Z. Tao, B. B. Toms, J. Goodisman and T. Asefa, Chem. Res. Toxicol., 2009, 22, 1869 CrossRef CAS PubMed.
- R. A. Petros and J. M. DeSimone, Nat. Rev. Drug Discovery, 2010, 9, 615 CrossRef CAS PubMed.
- D. F. Stoweand and A. K. S. Camara, Antioxid. Redox Signaling, 2009, 11, 1373 CrossRef PubMed.
- J. Cao, L. Jia, H. M. Zhou, Y. Liu and L. F. Zhong, Toxicol. Sci., 2006, 91, 476 CrossRef CAS PubMed.
- B. Li, H. He, B. B. Tao, Z. Y. Zhao, G. H. Hu, C. Luo, J. X. Chen, X. H. Ding, P. Sheng, Y. Dong, L. Zhang and Y.-C. Lu, Oncol. Lett., 2012, 28, 909 CAS.
- W. P. Roos and B. Kaina, Mol. Med., 2006, 12, 440 CAS.
- C. Riccardi and I. Nicoletti, Nat. Protoc., 2006, 1, 1458 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5bm00552c |
| This journal is © The Royal Society of Chemistry 2016 |