Facile synthesis of oxygen and sulfur co-doped graphitic carbon nitride fluorescent quantum dots and their application for mercury(II) detection and bioimaging†
Ya-Chun
Lu‡
a,
Jia
Chen‡
a,
Ai-Jun
Wang
a,
Ning
Bao
b,
Jiu-Ju
Feng
*a,
Weiping
Wang
a and
Linxiang
Shao
*a
aCollege of Chemistry and Life Science, College of Geography and Environmental Science, Zhejiang Normal University, Jinhua 321004, China. E-mail: jjfeng@zjnu.cn; shaolinxiang@zjnu.cn; Fax: +86-579-82282269; Tel: +86-579-82282269
bSchool of Public Health, Nantong University, Nantong 226019, China
First published on 28th October 2014
Abstract
In this work, uniform oxygen and sulfur co-doped graphitic carbon nitride quantum dots (OS-GCNQDs) have been prepared by thermal treatment of citric acid and thiourea. The as-obtained OS-GCNQDs show strong blue photoluminescence (PL) with a relatively high quantum yield of 14.5%. Furthermore, OS-GCNQDs exhibit stable and specific concentration-dependent PL intensities in the presence of mercury(II) ions in the range of 0.001–20.0 μM, with a detection limit of 0.37 nM (3S/N). More importantly, OS-GCNQDs were explored for cell imaging with satisfactory biocompatibility, and so are a potential fluorescent probe in biosensing and bioimaging applications.
Introduction
Carbon quantum dots (CQDs) have received growing research interest as promising carbon materials since they were discovered in 2004.1 Recently, many efforts have been focused on their prospective applications in optoelectronic devices,2 photocatalysis,3 electrocatalysis,4 biosensing, and bioimaging,5–7 owing to their superior properties, such as bright luminescence, good biocompatibility, and low toxicity.8,9 Specifically, their potential applications in the biological field are keenly anticipated. Therefore, intensive investigations have been carried out and numerous synthetic methods developed,10–12 such as electrochemical oxidation,13 ultrasonic treatment,14 microwave methods,15 solid thermal treatment,16 and hydrothermal methods.17Now, CQDs have broad applications in biochemical assays. Chen et al. synthesized CQDs from oil acid for cell imaging.18 Zhang's group fabricated CQDs via the oxidation of activated carbon by nitric acid. They also demonstrated selective and sensitive responses of CQDs to Cu2+.19 However, un-doped CQDs might have the disadvantage of self-quenching, thereby limit their further applications in bioanalysis.20 This is due to the intra-molecular ground-state dimer complex or energy transfer between the adjacent CQDs.
Alternatively, doped CQDs can almost retain all the advantages of blank CQDs and further avoid self-quenching phenomenon because of their substantial ensemble Stokes shift. Therefore, many researchers have paid much attention to doped CQDs with heteroatoms, especially nitrogen and sulfur.21–23 For cell imaging, our group synthesized N-doped CQDs (N-CQDs) by a thermal route from streptomycin.24 We also developed a solvent-free synthesis method to prepare SN-CQDs from glutathione for a highly selective and sensitive detection for mercury(II) ions.25 It needs to be emphasized that graphitic carbon nitride quantum dots (GNCQDs) are unique among heteroatom-doped CQDs as their structures are similar to graphene. They have broad applications in biomass conversion and sustainable chemistry, due to their functional groups.16,26,27
The mercury(II) ion (Hg2+) is one of the heavy metal ions widely used in industry and agriculture.28 Its strong toxicity and bioaccumulation result in serious human health problems even at very low concentrations.29 Therefore, developing a novel method for the trace detection of Hg2+ is very important. Conventional analytical approaches include atomic absorption/emission spectroscopy,30,31 Auger-electron spectroscopy,32 inductively coupled plasma mass spectrometry,33 ultraviolet-visible spectrometry,34 and polarography.35
In this work, we developed a facile one-step route for the synthesis of OS-GNCQDs from citric acid and thiourea (Scheme 1). The optical properties of the as-prepared OS-GNCQDs were examined in detail. Furthermore, OS-GNCQDs were explored for the selective and sensitive detection of Hg2+ as a model system.
Experimental
Materials
Citric acid and thiourea were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Mercuric nitrate, sodium nitrate, zinc sulfate heptahydrate, silver nitrate, manganese acetate tetrahydrate, copper nitrate trihydrate, nickel chloride hexahydrate, anhydrous magnesium sulfate, barium nitrate, aluminum nitrate nonahydrate, calcium nitrate tetrahydrate, cadmium chloride hemi(pentahydrate), ferric chloride, potassium chloride, iron(II) chloride tetrahydrate, and lead nitrate were received from Aladdin Chemistry Reagent Company (Shanghai, China). All the other chemicals were analytical grade and used as received. Twice-distilled water was used throughout all the experiments.Preparation of OS-GNCQDs
In a typical preparation of OS-GNCQDs (Scheme 1), 0.21 g citric acid and 0.23 g thiourea were mixed together under stirring (Fig. S1, ESI†). Next, the mixture was put into a 50 mL Teflon-lined autoclave, heated at 200 °C for 2.0 h (Fig. S2, ESI†), and cooled to room temperature in air. The product was dissolved with water, and re-purified with a 0.22 μM filter membrane to discard the nonfluorescent deposits, which were large particles with a flake-like structure. (Fig. S3, ESI†). The supernatant was collected and further diluted to prepare OS-GNCQD suspensions (5.0 mg mL−1).Instruments
UV-vis absorption spectra of the samples were recorded on a Lambda 950 UV-vis spectrophotometer (Perkin-Elmer, USA). Fluorescence emission spectroscopy was carried out on a LS-45 fluorescence spectrophotometer (Perkin-Elmer, UK). X-ray diffraction (XRD) measurements were performed on a Philips PW3040/60 automatic powder diffractometer using Cu Kα radiation. Fourier transform infrared spectra (FT-IR) were recorded on a Nicolet 670 FT-IR spectrometer in the form of KBr pellets. Transmission electron microscopy (TEM) and high resolution TEM (HR-TEM) images were taken on a JEOL-2100F transmission electron microscope. X-ray photoelectron spectra (XPS) were acquired on a Themo SCIENTIFIC ESCALAB 250 spectrometer with Al Kα X-ray radiation (1486.6 eV).Fluorescence detection of Hg2+
For a typical assay, 5.0 μL of OS-GNCQD suspension (5.0 mg mL−1) were diluted with 3.0 mL of water, followed by the addition of different concentrations of Hg2+. The mixed solutions were kept static, reacted for 20 min, and finally the associated fluorescence quenching spectra with an excitation wavelength of 369 nm were recorded at room temperature.Selectivity and interference measurements
The selectivity of OS-GNCQDs was examined using some interfering compounds such as Ag+, Al3+, Cd2+, Co2+, Cu2+, Fe2+, Fe3+, Mg2+, Mn2+, Na+, Ni2+, Pb2+, and Zn2+ under identical conditions. The concentrations of Hg2+ and the interferent ions were all 50.0 μM. For studying the interference, the OS-GNCQD suspension was mixed with Hg2+ in the absence and presence of other interferent chemicals at concentrations four times that of Hg2+. The associated fluorescence spectra were quickly recorded after incubation for 20 min.Cell imaging and toxicity assay
The cytotoxicity of OS-GNCQDs to human umbilical vein endothelial cells (HUVEC) was evaluated by a standard methylthiazolydiphenyltetrazolium bromide (MTT) assay. HUVEC were seeded in 96-well U-bottom plates at a density of 5.0 × 104–1.0 × 105 cells per milliliter (90.0 μL per well) and were initially cultured for 12 h in an incubator (37 °C, 5% CO2), prior to the addition of the OS-GNCQD suspension at a range of concentrations. After being cultured for a further 24 h with OS-GNCQDs, 20.0 μL of MTT solution (normal saline or 1.0 mg mL−1 phosphate buffer solution) was added to each sample, and incubated at 37 °C for 4 h. The culture media were discarded; then, 150.0 μL dimethylsulfoxide (DMSO) was added to each sample in order to dissolve the formazan, with shaking for at least 15 min. The corresponding spectra were recorded with a microplate reader at 570 nm. The cell viability rate (VR) was calculated based on the below equation:| VR (%) = A/A0 × 100% |
Results and discussion
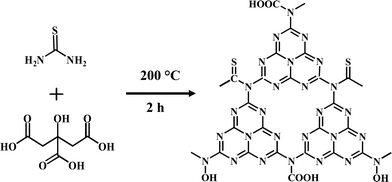
Fig. 1 shows the optical properties of OS-GNCQDs. Specifically, there is a UV-vis absorption peak centered at 338 nm, implying the presence of carbonyl or conjugated carbonyl groups. At the same time, the excitation and emission peaks appeared at 369 and 444 nm, respectively. In addition, the OS-GNCQD suspension exhibits a yellow color under visible light, whereas it is blue upon excitation with UV light of 365 nm (insets in Fig. 1A).As illustrated in Fig. 1B, the fluorescence spectra of the OS-GNCQDs shows a red shift by adjusting the excitation wavelength from 369 to 415 nm, accompanied by a fast decrease of the PL intensities. This indicates the excitation-dependent emission behavior of OS-GNCQDs, as supported by previous reports.7,18 Meanwhile, using quinine sulfate (54% in 0.1 mol L−1 H2SO4, λex = 369 nm) as a reference, the fluorescence quantum yield was calculated to be about 14.5% for OS-GNCQDs. This value is comparable to CQDs prepared from Bombyx mori silk,36 soya bean grounds,37 and ionic liquids,38 and fully consistent with previous research in which heteroatom-doped CQDs was found to enhance fluorescence dramatically.20,39
As shown by TEM images (Fig. 2), OS-GNCQDs have spherical shapes with an average size of 2.78 nm, similar to previous findings.3 Furthermore, well-defined lattice fringes are clearly observed (inset in Fig. 2A), with an inter-fringe distance of 0.202 nm, corresponding to the (102) diffraction planes of graphitic (sp2) carbon.40 This value matches well with CQDs using pemelo peel40 and natural gas soot41 as carbon sources. Fig. 2B reveals the narrow size distribution of OS-GNCQDs, with an average size of 2.75 nm.
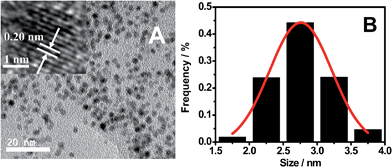 | ||
| Fig. 2 TEM image (A) and the respective size distribution (B) measured for 400 nanodots randomly. Inset shows high-resolution TEM image of an individual nanodot. | ||
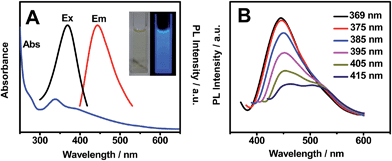
As displayed in Fig. 3A, there is a broad peak located at 27.0° and a weak one at 13.4° in the XRD pattern of OS-GNCQDs. The former represents the inter-planar graphitic stacking, and the latter is indicative of in-plane structural packing, reflecting the formation of graphitic carbon nitride.42,43
Meanwhile, FT-IR analysis was carried out to characterize the surface groups of OS-GNCQDs (Fig. 3B). The peaks emerge at 3415 and 3175 cm−1, which correspond to the stretching modes of N–H/O–H, suggesting highly hydrophilic property of OS-GNCQDs.3 The peak of 2380 cm−1 is ascribed to the stretching vibration of the S–H group, 2070 cm−1 to the C–N group,23 1670 cm−1 to the vibration absorption of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in COOH, 1410 cm−1 to the COO− group, and 1180–1080 cm−1 to C
O in COOH, 1410 cm−1 to the COO− group, and 1180–1080 cm−1 to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S vibrations.3,23 These results illustrate OS-GNCQDs with desirable functional groups.
S vibrations.3,23 These results illustrate OS-GNCQDs with desirable functional groups.
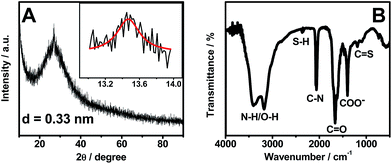
As revealed by the survey XPS spectrum of NSCDs (Fig. 4A), the typical product mainly contains O, N, C, S, and S elements. Specifically, the high-resolution C1s spectrum (Fig. 4B) shows five peaks detected at 284.2, 284.7, 285.3, 287.7, and 288.6 eV, which are attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–C/C–H, C–OH/C–O–C, C
C, C–C/C–H, C–OH/C–O–C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and COOH groups, respectively.44 And there are two peaks located at 398.9 and 399.6 eV in the high-resolution N1s region (Fig. 4C), which arise from pyridinic N and pyrrolic N,11 respectively. Similarly, two peaks emerge at 530.8 and 531.7 eV for the O1s spectrum (Fig. S4, ESI†), which originate from C–O and C
O, and COOH groups, respectively.44 And there are two peaks located at 398.9 and 399.6 eV in the high-resolution N1s region (Fig. 4C), which arise from pyridinic N and pyrrolic N,11 respectively. Similarly, two peaks emerge at 530.8 and 531.7 eV for the O1s spectrum (Fig. S4, ESI†), which originate from C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bands,45 respectively. Additionally, seven peaks are detected in the S2p spectrum (Fig. 4D), corresponding to –SH (161.8 eV), C–Sn–C (n = 1 or 2, 163.5 eV), –C
O bands,45 respectively. Additionally, seven peaks are detected in the S2p spectrum (Fig. 4D), corresponding to –SH (161.8 eV), C–Sn–C (n = 1 or 2, 163.5 eV), –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S– (164.8 eV), sulfoxide (166.2 eV), and –C–SOx– (x = 2, 167.7 eV; 3, 168.5 eV; 4, 169.3 eV),23,46 respectively. These phenomena demonstrate the formation of OS-GNCQDs in the present work.
S– (164.8 eV), sulfoxide (166.2 eV), and –C–SOx– (x = 2, 167.7 eV; 3, 168.5 eV; 4, 169.3 eV),23,46 respectively. These phenomena demonstrate the formation of OS-GNCQDs in the present work.
Fig. S5 (ESI†) illustrates the effects of pH values and salinity of the solutions on the PL intensities of OS-GNCQDs. The results verify a good stability of OS-GNCQDs in different physiological environments. Besides, OS-GNCQDs exhibit better photostability than traditional organic dyes such as rhodamine 6G. After three months storage at room temperature, OS-GNCQDs remain similar in PL intensity. These properties make OS-GNCQDs a potential fluorescent probe for biosensing and bioimaging.
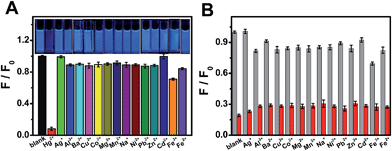
To estimate the selectivity of OS-GNCQDs as a fluorescent probe, the PL intensities of OS-GNCQDs were analyzed in the presence of metal ions at a concentration of 50.0 μM (Fig. 5A). It is found that fluorescence responses of OS-GNCQDs are different in the presence of different metal ions even under the same conditions. Impressively, the PL intensity of OS-GNCQDs can be greatly quenched in the case of Hg2+. Based on this phenomenon, OS-GNCQDs were efficiently used for the selective detection of Hg2+ in coexistence with other metal ions at a concentration of 200.0 μM (Fig. 5B). The outstanding selectivity and specificity can be ascribed to the stronger affinity of Hg2+ to amino groups and thiourea groups on the surface of OS-GNCQDs, relative to the affinities of other metal ions.
Fig. 6 shows the changes in high-resolution N and S spectra of OS-GNCQDs before and after the addition of Hg2+. The peak of N1s at 399.0 eV greatly decreases (Fig. 6A). This clearly demonstrates that Hg2+ has strong interactions with the pyridine nitrogen in OS-GNCQDs, which generate energy transfer from the OS-GNCQDs to Hg2+, leading to fluorescence quenching. Moreover, the sulfur peak at 162.6 eV significantly increases and the peak at 168.0 eV obviously decreases (Fig. 6B). This is ascribed to the formation of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S–Hg complexes based on the high affinity of thiourea groups to Hg2+. The as-formed complexes can facilitate electron transfer and restrain the radioactive recombination of excitations, leading to remarkable quenching effects on fluorescence intensity.47
S–Hg complexes based on the high affinity of thiourea groups to Hg2+. The as-formed complexes can facilitate electron transfer and restrain the radioactive recombination of excitations, leading to remarkable quenching effects on fluorescence intensity.47
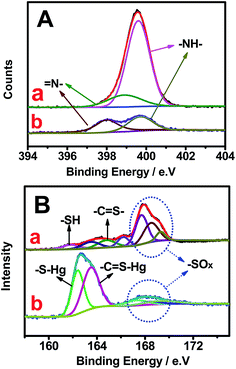 | ||
| Fig. 6 High-resolution N1s (A) and S2p (B) XPS spectra of OS-GCNQDs before (a) and after (b) the addition of Hg2+. | ||
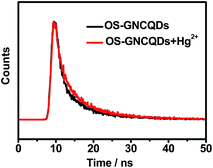
In addition, the PL lifetime of OS-GNCQDs is measured to be 7.88 ns, with excitation and emission wavelengths of 369 and 444 nm, respectively (Fig. 7). Nevertheless, the respective lifetime is decreased to 7.70 ns after the addition of Hg2+, mainly due to the static quenching that occurs in this case. Furthermore, this value is equal to that of CQDs prepared from streptomycin.24 This means that OS-GNCQDs can be used as a promising probe in biological analysis.
As described in Fig. 8, the PL intensities of OS-GNCQDs linearly decrease with Hg2+ concentrations. The quenching efficiency can be well fitted by the following Stern–Volmer equation:
| F0/F = 1 + KsvQ, |
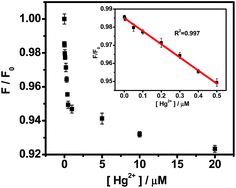 | ||
| Fig. 8 The relationship of the F/F0 and Hg2+ concentrations. The inset shows the linear range of 0.001–0.5 μM. The error bars represent variations among three independent measurements. | ||
Specifically, the PL intensities show linear responses to Hg2+ concentrations in the range of 0.001–0.5 μM (inset in Fig. 8). The detection limit is about 0.37 nM (S/N = 3) for Hg2+ detection. OS-GNCQDs have good sensitivity and a wide linear range, in contrast to those given in the literature, as listed in Table S1 (ESI†). The superior selectivity and sensitivity make OS-GNCQD a promising fluorescent probe in biomedical and environmental systems.
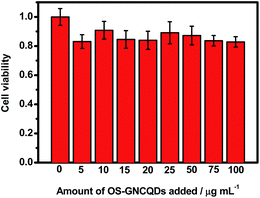
The MTT assay was conducted with HUVEC as a model cell to test the cytotoxicity of OS-GNCQDs, since cancer cells usually have more resistance to most chemical compounds. As depicted in Fig. 9, OS-GNCQDs exhibit good biocompatibility and low cytotoxicity for HUVEC, revealing a possible application of OS-GNCQDs for cell imaging.
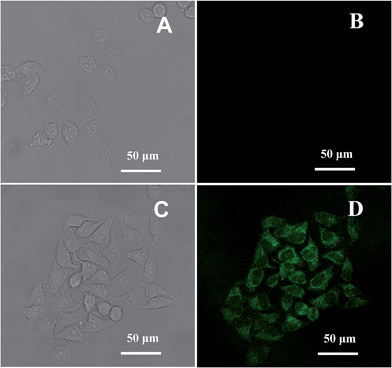
Fig. 10 shows confocal images of HeLa cells treated with OS-GNCQDs (75.0 μg mL−1). The fluorescence becomes brighter with the increase of the CQD concentrations, and vice versa (Fig. S6, ESI†). It is noteworthy that the concentration of OS-GNCQDs (75.0 μg mL−1) is much higher and the incubation time (24 h) is much longer for in vitro evaluation. These results indicate the better biocompatibility of OS-GNCQDs, which can be used for in vivo applications such as bioimaging.
Before incubation of HeLa cells with OS-GNCQD solution, there is no fluorescent response at a wavelength of 488 nm (Fig. 10A and B). After incubation, the cells in the cytoplasm become bright green under excitation at 488 nm. This means that CQDs can easily penetrate into the cytoplasm and label the cells simultaneously, illustrating the greatly improved fluorescence performance of OS-GNCQDs as a fluorescent probe in bioimaging.24,43,44 Therefore, OS-GNCQDs can potentially be applied for the investigation of labeled cytoplasm.
Conclusion
In summary, we report a simple route for synthesis of water-soluble OS-GNCQDs by a thermal treatment of citric acid and thiourea. The as-prepared uniform OS-GNCQDs possess a quantum yield of 14.5%, and have an average size of 2.78 nm, which is used for the selective and sensitive detection of Hg2+ with a detection limit of 0.37 nM. Furthermore, OS-GNCQDs were explored for bioimaging with improved biocompatibility.Acknowledgements
This work was financially supported by National Natural Science Foundation of China (nos 21475118, 21175118, 21275130, 21305128 and 21345006), and Zhejiang province university young academic leaders of academic climbing project (no pd2013055).References
- X. Xu, R. Ray, Y. Gu, H. J. Ploehn, L. Gearheart, K. Raker and W. A. Scrivens, J. Am. Chem. Soc., 2004, 126, 12736–12737 CrossRef CAS PubMed.
- D. Zhang, Y. Hao, L. Zheng, Y. Ma, H. Feng and H. Luo, J. Mater. Chem. A, 2013, 1, 7584–7591 CAS.
- D. Qu, M. Zheng, P. Du, L. Zhang, Y. Zhou, D. Li, H. Tan, Z. Zhao, Z. Xie and Z. Sun, Nanoscale, 2013, 5, 12272–12277 RSC.
- Z. Liu, H. Nie, Z. Yang, J. Zhang, Z. Jin, Y. Lu, Z. Xiao and S. Huang, Nanoscale, 2013, 5, 3283–3288 RSC.
- K. Qu, J. Wang, J. Ren and X. Qu, Chem.–Eur. J., 2013, 19, 7243–7249 CrossRef CAS PubMed.
- A. Salinas-Castillo, M. Ariza-Avidad, C. Pritz, M. Camprubi-Robles, B. Fernandez, M. J. Ruedas-Rama, A. Megia-Fernandez, A. Lapresta-Fernandez, F. Santoyo-Gonzalez, A. Schrott-Fischer and L. F. Capitan-Vallvey, Chem. Commun., 2013, 49, 1103–1105 RSC.
- L. Shen, L. Zhang, M. Chen, X. Chen and J. Wang, Carbon, 2013, 55, 343–349 CrossRef CAS PubMed.
- J. C. G. Esteves da Silva and H. M. R. Gonçalves, Trends Anal. Chem., 2011, 30, 1327–1336 CrossRef CAS PubMed.
- P. G. Luo, S. Sahu, S.-T. Yang, S. K. Sonkar, J. Wang, H. Wang, G. E. LeCroy, L. Cao and Y.-P. Sun, J. Mater. Chem. B, 2013, 1, 2116–2127 RSC.
- M. Tan, L. Zhang, R. Tang, X. Song, Y. Li, H. Wu, Y. Wang, G. Lv, W. Liu and X. Ma, Talanta, 2013, 115, 950–956 CrossRef CAS PubMed.
- Z. Qian, J. Ma, X. Shan, L. Shao, J. Zhou, J. Chen and H. Feng, RSC Adv., 2013, 3, 14571–14579 RSC.
- X. Wang, K. Qu, B. Xu, J. Ren and X. Qu, Nano Res., 2011, 4, 908–920 CrossRef CAS PubMed.
- Y.-L. Zhang, L. Wang, H.-C. Zhang, Y. Liu, H.-Y. Wang, Z.-H. Kang and S.-T. Lee, RSC Adv., 2013, 3, 3733–3738 RSC.
- S. Zhuo, M. Shao and S.-T. Lee, ACS Nano, 2012, 6, 1059–1064 CrossRef CAS PubMed.
- Y. Liu, N. Xiao, N. Gong, H. Wang, X. Shi, W. Gu and L. Ye, Carbon, 2014, 68, 258–264 CrossRef CAS PubMed.
- J. Zhou, Y. Yang and C.-Y. Zhang, Chem. Commun., 2013, 49, 8605–8607 RSC.
- W. Li, Z. Zhang, B. Kong, S. Feng, J. Wang, L. Wang, J. Yang, F. Zhang, P. Wu and D. Zhao, Angew. Chem., Int. Ed., 2013, 52, 1–6 CrossRef.
- B. Chen, F. Li, S. Li, W. Weng, H. Guo, T. Guo, X. Zhang, Y. Chen, T. Huang, X. Hong, S. You, Y. Lin, K. Zeng and S. Chen, Nanoscale, 2013, 5, 1967–1971 RSC.
- S. Zhang, Q. Wang, G. Tian and H. Ge, Mater. Lett., 2014, 115, 233–236 CrossRef CAS PubMed.
- P. Wu and X.-P. Yan, Chem. Soc. Rev., 2013, 42, 5489–5521 RSC.
- W. Shi, X. Li and H. Ma, Angew. Chem., 2012, 124, 6538–6541 CrossRef.
- S. Chandra, P. Patra, S. H. Pathan, S. Roy, S. Mitra, A. Layek, R. Bhar, P. Pramanik and A. Goswami, J. Mater. Chem. B, 2013, 1, 2375–2382 RSC.
- D. Sun, R. Ban, P.-H. Zhang, G.-H. Wu, J.-R. Zhang and J.-J. Zhu, Carbon, 2013, 64, 424–434 CrossRef CAS PubMed.
- W. Wang, Y.-C. Lu, H. Huang, J.-J. Feng, J.-R. Chen and A.-J. Wang, Analyst, 2014, 139, 1692–1696 RSC.
- W. Wang, Y.-C. Lu, H. Huang, A.-J. Wang, J.-R. Chen and J.-J. Feng, Sens. Actuators, B, 2014, 202, 741–747 CrossRef CAS PubMed.
- Y. Wang, X. Wang and M. Antonietti, Angew. Chem., Int. Ed., 2012, 51, 68–89 CrossRef CAS PubMed.
- Y. Zhang, J. Liu, G. Wu and W. Chen, Nanoscale, 2012, 4, 5300–5303 RSC.
- R. Liu, H. Li, W. Kong, J. Liu, Y. Liu, C. Tong, X. Zhang and Z. Kang, Mater. Res. Bull., 2013, 48, 2529–2534 CrossRef CAS PubMed.
- R. Zhang and W. Chen, Biosens. Bioelectron., 2014, 55, 83–90 CrossRef CAS PubMed.
- J. Margetínová, P. Houserová-Pelcová and V. Kubáň, Anal. Chim. Acta, 2008, 615, 115–123 CrossRef PubMed.
- S. Gil, I. Lavilla and C. Bendicho, Spectrochim. Acta B, 2007, 62, 69–75 CrossRef PubMed.
- M. Miró and E. H. Hansen, Anal. Chim. Acta, 2013, 782, 1–11 CrossRef PubMed.
- A. Castillo, A. F. Roig-Navarro and O. J. Pozo, Anal. Chim. Acta, 2006, 577, 18–25 CrossRef CAS PubMed.
- H. Bagheri and A. Gholami, Talanta, 2001, 55, 1141–1150 CrossRef CAS.
- S. Z. Milić, N. I. Potkonjak, S. Ž. Gorjanović, S. D. Veljović-Jovanović, F. T. Pastor and D. Ž. Sužnjević, Electroanalysis, 2011, 23, 2935–2940 CrossRef.
- Z. L. Wu, P. Zhang, M. X. Gao, C. F. Liu, W. Wang, F. Leng and C. Z. Huang, J. Mater. Chem. B, 2013, 1, 2868–2873 RSC.
- W. Li, Z. Yue, C. Wang, W. Zhang and G. Liu, RSC Adv., 2013, 3, 20662–20665 RSC.
- A. Zhao, C. Zhao, M. Li, J. Ren and X. Qu, Anal. Chim. Acta, 2014, 809, 128–133 CrossRef CAS PubMed.
- C. Liu, P. Zhang, F. Tian, W. Li, F. Li and W. Liu, J. Mater. Chem., 2011, 21, 13163–13167 RSC.
- W. Lu, X. Qin, S. Liu, G. Chang, Y. Zhang, Y. Luo, A. M. Asiri, A. O. Al-Youbi and X. Sun, Anal. Chem., 2012, 84, 5351–5357 CrossRef CAS PubMed.
- L. Tian, D. Ghosh, W. Chen, S. Pradhan, X. Chang and S. Chen, Chem. Mater., 2009, 21, 2803–2809 CrossRef CAS.
- Q. Su, J. Sun, J. Wang, Z. Yang, W. Cheng and S. Zhang, Catal. Sci. Technol, 2014, 4, 1556–1562 CAS.
- G. Zhang, J. Zhang, M. Zhang and X. Wang, J. Mater. Chem., 2012, 22, 8083–8091 RSC.
- S. Sahu, B. Behera, T. K. Maiti and S. Mohapatra, Chem. Commun., 2012, 48, 8835–8837 RSC.
- J. Wei, J. Shen, X. Zhang, S. Guo, J. Pan, X. Hou, H. Zhang, L. Wang and B. Feng, RSC Adv., 2013, 3, 13119–13122 RSC.
- Y. Su, Y. Zhang, X. Zhuang, S. Li, D. Wu, F. Zhang and X. Feng, Carbon, 2013, 62, 296–301 CrossRef CAS PubMed.
- H. Huang, J.-J. Lv, D.-L. Zhou, N. Bao, Y. Xu, A.-J. Wang and J.-J. Feng, RSC Adv., 2013, 3, 21691–21696 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4tc02111h |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |