Formation of double helical microfibrils from small molecules†
Xianbin
Jia‡
ac,
Shiyun
Lou‡
a,
Honglei
Yuan‡
b,
Ruijian
Yuan
d,
Shasha
Tian
a,
Chunyu
Niu
a,
Xinjuan
Li
c and
Shaomin
Zhou
*a
aKey Lab for Special Functional Materials of Ministry of Education, Henan University, Kaifeng 475004, China. E-mail: smzhou@henu.edu.cn
bDepartment of Physics, Southeast University, Nanjing 211189, China
cSchool of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang 453007, China
dSupervision and Inspection Centre for Packaging Printing Product, Tongcheng, 231400, China
First published on 27th August 2014
Abstract
Using a facile vapor–solid route, double helical, organic, small molecular microfibril, i.e. 3,4,9,10-perylenetetracarboxylic dianhydride (PTCDA) was synthesized, which was based on the spontaneous twisting of supramolecular microtubes. The average diameter of the resulting helical structures was about 200 nm and the overall length was of several micrometers. The helical microfibrils, which were obtained by coiling the multilayer microtubes from the inner to the outer molecular layers, tend to release their internal rotation stress to exhibit the most stable morphology as indicated by a series of characterizations. The change in van der Waals force, together with the surface free energy among the adjacent microtubes molecular layers, were the driving forces to induce the formation of a helical structure. The obtained results present an extremely facile strategy for the fabrication of small molecular, double helical microfibrils with morphological transformation.
Introduction
Helix is prevalent in both living and non-living systems. In the research field of biology, helix is a central structural motif, which ranges from nanoscopic DNA double helix, collagen triple helix to microscopic viruses, bacteria, macroscopic conch, escargots, and galaxy etc.1–3 Materials with a double-helix structure have attracted intensive attention due to their elegant morphology and their amazing morphology-related potential applications.4–6 Scientists have long held a fascination, sometimes bordering on mystical obsession for helical structures. Rotational symmetry, chirality and morphology-related applications are only a few attributes associated with a helix, which make it special for materials science.7,8 Inspired by such sophisticated structures, chemists have been challenged to mimic its unique form and have explored various helical structures by self-assembly at the micro-nanoscale.9–11 On the basis of interactions such as metal–ligand interactions,12 H-bonds,13 aromatic–aromatic interactions14 or π–π interactions,15 different kinds of double helical microstructures have been developed. To date, most of the research on materials with helical structures has been focused on macromolecules and inorganics, and less attention has been paid to organic conjugated small molecules, although the low-molecular-mass organic materials have a lot of advantages over their inorganic counterparts due to their promising applications in electronic and optoelectronic micro-/nano-devices.16–18 The helical structure is a complex structure, the formation of such a structure requires special conditions, thus it is difficult to control the structure and interactions of small molecule monomers.19,203,4,9,10-Perylenetetracarboxylic dianhydride (PTCDA) is an archetypal organic semiconducting, conjugated small molecule, which is a potential building block for the realization of organic electronic devices.21,22 In our previous study (J. Mater. Chem., 2012, 22, 883), PTCDA microtubes (MTs) were prepared, which were obtained from the curling and seaming of a two-dimensional (2D) lamellar structure, constructed with the cooperation of some noncovalent interactions such as π–π interactions and H-bonds. To the best of our knowledge, reports on microscale materials with helical architectures have rarely been witnessed, especially through physical methods. In this study, we demonstrate that well-defined double helical microfibrils can be readily fabricated, which undergo a shape transformation process based on the spontaneous twisting of PTCDA microtubes by a simple physical vapour deposition (PVD) on silicon substrates. This is a completely new phenomenon observed in organic small molecule nanostructures. The process of evolution of helical microfibrils was demonstrated by observation using scanning electron microscopy (SEM) and transmission electron microscopy (TEM), which contributed to experimental evidence and made it possible to view the mechanism of formation. In addition, it is particularly important that alcohol is one kind of central nervous system depressant, and a higher concentration of alcohol in the human body can cause lack of timely response measures. The effective and efficient monitoring of the concentration of alcohol to avoid unfavourable circumstances is the need of the hour. Based on the PVD technique, we have fabricated PTCDA double helical microfibrils, which have good sensitivity in low concentrations of alcohol at room temperature. We believe that the gas sensors may have promising applications in the future and stimulate research for other kinds of nanostructured gas sensors.
Results and discussion
By the thermal evaporation method, the helical microfibrils were deposited on a silicon substrate. The substrate, together with a quartz boat on which PTCDA powders (20 mg) in an Ar ambient at a vapor-flow rate of 50 sccm (standard cubic centimetres per minute), were put into a quartz boat (closed at both ends). The typical growth process was conducted at 550 °C for 50 min, followed by condensation of the vapor on the substrate at a temperature of 410 °C, and then the substrate was cooled at a rate of 4 °C min−1. A high density of helical structures can be seen in the representative overview SEM image (Fig. 1). Studies on the transmission electron microscopy (TEM) images (Fig. 2) revealed that both left- (Fig. 2a) and right-handed (Fig. 2b) double helical microfibrils existed in the sample; moreover, in each helix, the chirality was always consistent. These superstructures (Fig. 2c) had diameters of approximately 200 nm and a pitch of 150 nm and an extended length of several micrometers.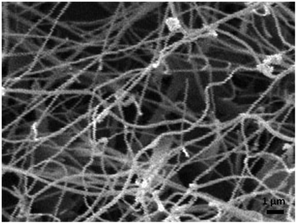 | ||
| Fig. 1 Representative overview SEM image of the as-synthesized PTCDA double helical microfibrils deposited on the silicon substrate. | ||
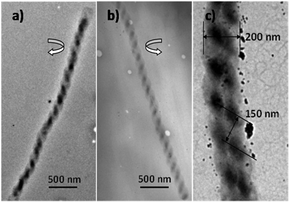 | ||
| Fig. 2 TEM images of PTCDA double helical microfibrils, showing different chirality: (a) left-handed, (b) right-handed, (c) diameters and pitch of the helical structure. | ||
The dominating factors, which are crucial to the growth of PTCDA helical microfibrils, are the sublimation temperature of the PTCDA molecules and the silicon substrate temperature. The preferential formation of microtubes instead of other microstructures such as microwires is achieved when the sublimation temperature of the PTCDA is 500–550 °C, and the temperature of the substrates (TOS) and the sublimation temperature of the PTCDA molecules (TS) complies with the expression: 0.65 TS < TOS ≤ 0.7 TS, as shown in Fig. S2.† When the deposition process is carried out at a TS close to 550 °C and TOS >0.7 TS, the PTCDA molecules tend to form a one-dimensional (1D) helical structure on the substrate.
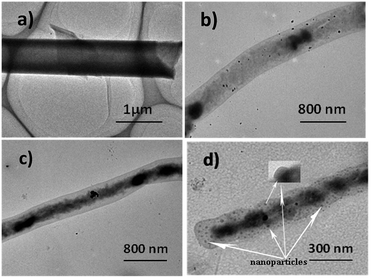
The growth morphology of the microstructures can be affected by the temperature of the substrate. A vertically positioned substrate was used, so that the temperature across the entire substrate would be constant in the tube furnace. In order to better understand the process of formation of well-defined double helical microfibrils, the sublimation temperature of the PTCDA molecules during the deposition was maintained at 550 °C, and the corresponding transformations of morphology with four TOS [about 360 °C (Fig. 3a), 380 °C (Fig. 3b), 400 °C (Fig. 3c) and 410 °C (Fig. 3d)] for TEM observation are presented in Fig. 3a–d. It is obvious that different morphologies are formed in the four substrates. As shown in Fig. 3a, microtubes with diameters of approximately 1 μm and lengths up to several micrometers can be synthesized at a substrate temperature of 360 °C. According to our previous study (J. Mater. Chem., 2012, 22, 883), these PTCDA microtubes are constructed via the curling and seaming of a 2D lamellar structure, which was assembled by the layer-by-layer technique. This result implies that PTCDA microtubes together with the tubular structures deposition process on glass substrates follow a general similar folding behaviour which can be obtained via physical vapour deposition process. Similar results are also found in other small organic molecules materials. Similar results are also found in other small organic molecule materials.18,23 The increase in the TOS (380 °C) can result in a dramatic change in the shape of the PTCAD tubes. The TEM image (Fig. 3b) of the sample revealed that the inner conformation of the PTCDA microtubes leads to conglomeration. When the TOS was upgraded to 400 °C, it was observed that the inner space of the as-grown PTCDA microtubes continued to grow spirally (Fig. 3c). During this process, the diameter of the microtubes significantly decreased from approximately 1 μm to 0.5 μm. It was the sequential morphological transformation, which provided strong evidence that PTCDA microtubes were formed via the assembly of a 2D lamellar structure by the cooperation of noncovalent interactions such as π–π interactions and H-bonds. It is reasonable to assume that the contraction of inner space induces the geometrical curvature required for twisting its shape and to make the whole system stable. The distorted multilayer lamellar microtubes were merely intermediates in the formation of the final double helical microfibrils, which further evolved into PTCDA microtubes by growth on the silicon substrate at 410 °C (Fig. 3d). The as-grown products have a diameter ranging from 200 to 300 nm and a length longer than 10 μm.
It is well known that the formation of organic micro- and nano-structures with a different morphology is determined by different dominant intermolecular interactions. These are noncovalent forces such as H-bonds, π–π interactions, and van der Waals force, which play decisive roles in the formation of organic low-dimensional structures. By PVD methods, many inorganic helical micro-materials such as helical carbon, SiO2, ZnO, and MgB2,24–30 have been prepared at high temperatures. While significant progress has been made in assembling small molecules to form supramolecular helical microstructures via multiple noncovalent interactions, success has been limited by the chemical techniques involved in the process. Similar right- and left-handed twisted microfibers have also been prepared by the polymerization of aniline with ammonium persulfate in water in the presence of D- and L-CSAs as the dopant. Yan et al. believed that helical polyanilines self-assemble to form superhelices with a helical bias, which originate from the polyaniline helicity induced by the dopants.31 The present case could be different, because no microscopic chirality at the atomic scale exists, and PTCDA has a planar molecular structure, which cannot form macroscopic double-helical morphologies without introducing defects due to supramolecular assembly. For our experiment, the strong π–π interaction between PTCDA molecules was not damaged during the conversion of PTCDA microtubes into helical microfibrils. However, we do not believe that π–π interaction is the only force responsible for the helical structure formation. For example, in order to reach thermodynamic equilibrium states by such morphology evolution, the van der Waals force together with the surface free energy change taking place in the vapor–solid process may play a crucial role.
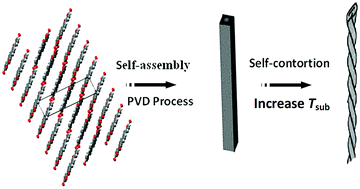
The experimental results indicate that the double helical microfibrils experience a self-twisting procedure. The possible mechanism of helical formation is discussed (as indicated in Fig. 4). Experimental procedure showed that different TOS, the microtube with different morphologies, accompanied by an increase in the surface free energy, causes a change in the van der Waals force between the PTCDA monolayer sheets, and facilitates the formation of layer-layer loose microtubes. Therefore, we speculate that this change in van der Waals force among adjacent PTCDA molecular layers is the primary driving force for the double-helical structure formation. The as-grown loose multilayer microtubes with the same-handed rotation coil on them release the internal stress and achieve a more stable morphology from the inner to outer molecule layers. This leads to a reduction in the microtube diameter, which competes with the increase in surface free energy for reaching equilibrium states, thereby suggesting that the twisted tubes in a helical conformation can distribute the energy effectively. The PTCDA double-helical microstructure can be achieved by a balance between the van der Waals force and the altered surface free energy among the adjacent microtubes molecular layers. Such a unique and complicated driving force could also be responsible for the fleet formation process. Morphology evolution is very similar to the formation of double helical Si microtubes, but it is different from the formation of the Si double-helix by a balance between the inner pressure of the melt and the viscosity of the melt.32 However, an in-depth investigation of the mechanism of the helical microstructure formation is still in progress.
As far as we know, Au is known for forming ohmic contact with p-type organic semiconductors, because the work function of the metal is close to or larger than the sum of the electron affinity and the bandgap energy. It can be seen that both curves have a linear relationship with the variation in the applied voltage, which proves that good ohmic contacts are achieved for Au/PTCDA/Au. Room-temperature measurement processing (MP) shows linear I–V characteristics, whereas the high-temperature MP significantly alters the contact properties and effectively changes ohmic contact to Schottky contact.33 When the PTCDA double helical microfibrils were exposed to air and different concentrations of ethanol at room temperature, the resistance in the air (Ra) and in the test gas (Rg) (as shown in Table 1) are obtained by combining the DC voltage (−10 to +10 V) on the material mounted on gold electrodes, and the SEM image of the PTCDA microfibrils embedded into the two Au electrodes at both ends in the test device is shown as Fig. 5, which indicates that the ohmic contacts have been achieved as the previous work of our group.34
| Concentration of alcohol (ppm) | 0 | 5 | 10 | 20 |
|---|---|---|---|---|
| R a(×105) | 1.51 | 1.53 | 1.55 | 1.57 |
| R g(×105) | — | 5.17 | 6.76 | 7.61 |
| R a/Rg | — | 3.38 | 4.36 | 4.85 |
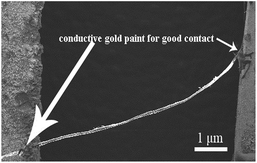 | ||
| Fig. 5 The representative overview SEM image of the PTCDA double helical microfibrils on the gold electrode. | ||
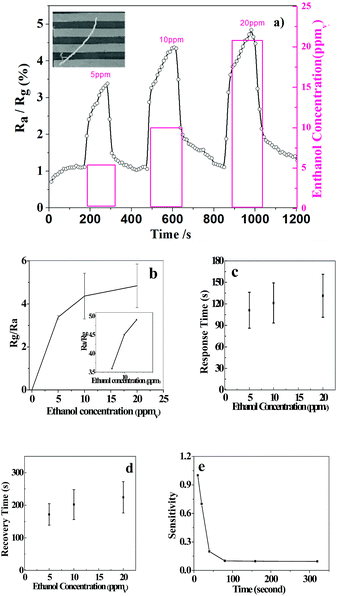
Due to the large ratio of surface area to volume and unique microstructure, most microfibers have good gas sensitivity, especially for the detection of ethanol. The gas sensing properties were measured by mounting the device in a casing having a volume of 300 cm3. The resistance variations were recorded with a Keithley 4200-SCS system on exposure to ethanol vapor. Fig. 6a shows the typical response curves when the single PTCDA double helical microfibrils were exposed to different concentrations of ethanol at room temperature. When an ethanol vapour of 5–20 ppmv is introduced into and removed from the testing atmosphere, the response of the single PTCDA microfibrils-based sensors increases drastically and decreases rapidly in each cycle. The present PTCDA microstructures showed a remarkable response (S = 4.85) at 20 ppm ethanol vapour. In Fig. 6b, the response calibration curves of the as-obtained products are indicated. Moreover, the double helical microfibrils-based sensor offers a fine response (Fig. 6c), recovery time (Fig. 6d), and sensitivity of sensor resistance at room temperature (Fig. 6e). The enhancement of the ethanol gas response should be understood in relation to the electron donation from ethanol through electron-donor–electron-acceptor complexation with PTCDA molecules. We believe that such π–π stacking interactions may strongly change the molecular properties such as exciton and charge transport, which are of importance for applications of π-conjugated molecules in the design of novel organic optoelectronic devices and sensors. Fig. 7 shows the TEM images of the fibrils and tubes before (Fig. 7a) and after (Fig. 7b) adsorption of ethanol. From these images, we could perceive that there was no effect on the morphology mainly due to the pure physical change instead of a chemical reaction (inertness of ethanol), indicating good reproducibility and stability.
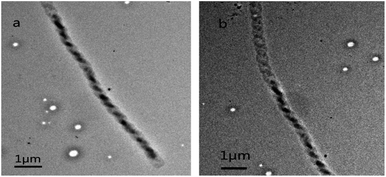 | ||
| Fig. 7 Representative overview TEM image of the as-synthesized PTCDA double helical microfibrils deposited on the silicon substrate (a) before adsorption and (b) after adsorption. | ||
Conclusions
In summary, we demonstrate for the first time that supramolecular PTCDA microtubes can self-twist into well-defined double helical microstructures with the PVD method. Studies on the controlling factors revealed that TOS played a crucial role in the growth of PTCDA helical microfibrils. The evolution process of the PTCDA double helical structures was demonstrated by observation of the SEM and TEM images, which provided experimental evidence for the origin of double helical microfibrils, accomplished by the twisting of PTCDA microtubes. The formation of the double helical microfibrils may be realized through a balance complex interplay between van der Waals force among adjacent PTCDA molecule layers and free energy, as well as π–π interactions on supramolecular microtubes. This work may provide a new alternative approach to the design and construction of helical microstructures and well-ordered, one-dimensional, organic small molecule assemblies.Experimental section
Preparation of PTCDA microfibrils
The PTCDA microfibrils were fabricated by a physical vapour deposition method. PTCDA powder was purchased from Sigma-Aldrich and was used without further treatment. In a typical preparation, a quartz boat loaded with the PTCDA powder was placed into the centre of a quartz tube, which was inserted into a horizontal tube furnace. The vapourizing temperature of PTCDA was maintained at 550 °C at a heating rate of 20 °C min−1 under Ar flow of 50 sccm, controlled by a needle valve and measured using a rotameter. The silicon substrates were thoroughly rinsed with acetone and ethanol, dried with N2 and placed along the downstream side of the flowing Ar to collect the products, and the substrate temperature was measured using a thermocouple. After 50 min, the substrates were cooled at a rate of 4 °C min−1, and the PTCDA source was physically deposited onto the substrates, yielding some wool-like products.Characterization
The size and morphology of the as-prepared PTCDA double helix were investigated by using scanning/transmission electron microscopy (SEM/TEM, JSM5600LV/JEOL2010).Device fabrication and characterization
PTCDA microfibrils-based device for resistance (R)–time (t) measurement was assembled on Au electrodes by dielectrophoresis. For dielectrophoresis, a DC voltage with 10 V and 50 kHz was applied to the Au electrode. The response of the sensors to ethanol was measured by mounting the device in a casing having a volume of 300 cm3. The resistance variations were recorded with a Keithley 4200-SCS system on exposure to ethanol vapor. The sensor sensitivity to ethanol gas is defined as S = Ra/Rg, where Ra and Rg are the resistance in air and in test gas, respectively.Acknowledgements
This work is partially financially supported by the National Natural Science Foundation of China (Grant 1371049, 20971036, 21204019 and 20604008) and the Changjiang Scholars and innovative Research Team in University, no. PCS IRT1126.Notes and references
- J. J. Cornelissen, Science, 1998, 280, 1427 CrossRef CAS.
- A. P. Slobozhanyuk, M. Lapine, D. A. Powell, I. V. Shadrivov, Y. S. Kivshar, R. C. McPhedran and P. A. Belov, Adv. Mater., 2013, 25, 3409 CrossRef CAS PubMed.
- Y. Qiao, Y. Y. Lin, Y. J. Wang, Z. Y. Yang, J. Liu, J. Zhou, Y. Yan and J. Huang, Nano Lett., 2009, 9, 4500 CrossRef CAS PubMed.
- F. Nitze, E. A. Hamad and T. Wågberg, Carbon, 2011, 49, 1101 CrossRef CAS PubMed.
- O. O. Ogunro and X. Q. Wang, Nano Lett., 2009, 9, 1034 CrossRef CAS PubMed.
- D. S. Su, Angew. Chem., 2011, 50, 4747 CrossRef CAS PubMed.
- E. Yashima, K. Maeda and Y. Furusho, Acc. Chem. Res., 2008, 41, 1166 CrossRef CAS PubMed.
- D. S. Su, Angew. Chem., Int. Ed., 2011, 50, 4747 CrossRef CAS PubMed.
- J. J. Cornelissen, Science, 1998, 280, 1427 CrossRef CAS.
- Q. Zhang, M. Q. Zhao, D. M. Tang, F. Li, J. Q. Huang, B. Liu, W. C. Zhu, Y. H. Zhang and F. Wei, Angew. Chem., Int. Ed., 2010, 49, 3642 CrossRef CAS PubMed.
- V. Kitaev, J. Mater. Chem., 2008, 18, 4745 RSC.
- H. J. Kim, E. Lee, M. G. Kim, M.-C. Kim, M. Lee and E. Sim, Chem.–Eur. J., 2008, 14, 3883 CrossRef CAS PubMed.
- J. Li, J. A. Wisner and M. C. Jennings, Org. Lett., 2007, 9, 3267 CrossRef CAS PubMed.
- H. Goto, Y. Furusho, K. Miwa and E. Yashima, J. Am. Chem. Soc., 2009, 131, 4710 CrossRef CAS PubMed.
- S. L. Lee, N. T. Lin, W. C. Liao, C. h. Chen, H. C. Yang and T. Y. Luh, Chem.–Eur. J., 2009, 15, 11594 CrossRef CAS PubMed.
- Z. F. Li, Q. F. Dong, Y. W. Li, B. Xu, M. Deng, J. N. Pei, J. B. Zhang, F. P. Chen, S. P. Wen, Y. J. Gao and W. J. Tian, J. Mater. Chem., 2011, 21, 2159 RSC.
- L. Zhang, H. Li, C. S. Ha, H. Suh and I. Kim, Langmuir, 2010, 26, 17890 CrossRef CAS PubMed.
- Y. S. Zhao, W. Yang, D. Xiao, X. Sheng, X. Yang, Z. Shuai, Y. Luo and J. Yao, Chem. Mater., 2005, 17, 6430 CrossRef CAS.
- H. Liu, Y. Li, S. Xiao, H. Gan, T. Jiu, H. Li, L. Jiang, D. Zhu, D. Yu, B. Xiang and Y. Chen, J. Am. Chem. Soc., 2003, 125, 10794 CrossRef CAS PubMed.
- L. Zang, Y. Che and J. S. Moore, Acc. Chem. Res., 2008, 41, 1596 CrossRef CAS PubMed.
- L. Chen, H. Li and A. Wee, Phys. Rev. Lett., 2010, 105, 226103 CrossRef.
- M. Mura, X. Sun, F. Silly, H. T. Jonkman, G. A. D. Briggs, M. R. Castell and L. N. Kantorovich, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 81, 195412 CrossRef.
- Y. Tian, Q. He, C. Tao and J. B. Li, Langmuir, 2006, 22, 360 CrossRef CAS PubMed.
- X. Zhang, X. Zhang, W. Shi, X. Meng, C. S. Lee and S. T. Lee, Angew. Chem., Int. Ed., 2007, 46, 1525 CrossRef CAS PubMed.
- F. Nitze, E. Abou-Hamad and T. Wågberg, Carbon, 2011, 49, 1101 CrossRef CAS PubMed.
- P. X. Gao, Y. Ding, W. J. Mai, W. L. Hughes, C. S. Lao and Z. L. Wang, Science, 2005, 309, 1700 CrossRef CAS PubMed.
- H. F. Zhang, C. M. Wang, E. C. Buck and L. S. Wang, Nano Lett., 2003, 3, 577 CrossRef CAS.
- M. Nath and B. A. Parkinson, J. Am. Chem. Soc., 2007, 129, 11302 CrossRef CAS PubMed.
- M. Q. Zhao, Q. Zhang, G. L. Tian, J. Q. Huang and F. Wei, ACS Nano, 2012, 6, 4520 CrossRef CAS PubMed.
- M. Yang and N. A. Kotov, J. Mater. Chem., 2011, 21, 6775 RSC.
- Y. Yan, Z. Yu, Y. W. Huang, W. X. Yuan and Z. X. Wei, Adv. Mater., 2007, 19, 3353 CrossRef CAS.
- H. Morito and H. Yamane, Angew. Chem., Int. Ed., 2010, 49, 3638 CrossRef CAS PubMed.
- M. Tahir, M. H. Sayyad, F. Wahab, D. N. Khan and F. Aziz, Phys. B, 2013, 415, 77 CrossRef CAS PubMed.
- X. B. Tang, G. M. Li and S. M. Zhou, Nano Lett., 2013, 13, 5046 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4tc01083c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |