Understanding the pathway of antibacterial activity of copper oxide nanoparticles†
Surapaneni Meghana‡
a,
Prachi Kabra‡a,
Swati Chakrabortyb and
Nagarajan Padmavathy*c
aSchool of Biosciences and Technology, VIT University, India
bJadavpur University, Kolkata, India
cMaterials Chemistry Division, School of Advanced Sciences, VIT University, Vellore – 632014, Tamilnadu, India.. E-mail: pdyvit@gmail.com
First published on 23rd December 2014
Abstract
This work investigates the role of oxidation state in the antibacterial activity of copper oxide nanoparticles (NPs). The findings add strong support to a contact killing mechanism of copper oxides (CuO and Cu2O) through which bacteria initially suffer severe damage to the cell envelope. Then further damage ensues by an independent pathway of each copper oxide nanoparticle. Formation of copper(I)–peptide complex from cuprous oxide (Cu2O) and free radical generation from cupric oxide (CuO) were identified as key sources of toxicity towards E.coli. Cu2O rapidly inactivated Fumarase A, an iron sulphur cluster enzyme suggesting the cuprous state of copper binding to the proteins. This inactivation was not noticed in CuO. The percentage of biocidal/bacteriostatic activity is closely related to the oxidation state of the copper oxides. In the case of E.coli, Cu2O nanoparticles showed more efficient antibacterial activity and higher affinity to the bacterial cells. CuO nanoparticles produced significant ROS in terms of super oxides while Cu2O did not. The diminishing defective emission peaks of Cu2O after incubation with microbes strongly suggest the formation of protein complexes. This work is carried out to enable better understanding of the mechanistic pathways of copper oxide nanoparticles.
Introduction
Nanostructured materials such as silver, copper, ZnO, MgO, TiO2, CuO, carbon nanotubes (CNTs) and their composites possessing antibacterial properties have recently received much attention.1 The use of such nanomaterials in medical devices is to prevent bacterial infection.2 Elemental copper and its compounds have been recognized as antimicrobial materials by the US Environmental Protection agency (EPA).3 Copper(I and II) oxides in its nanoform (<100 nm) displays enhanced antimicrobial activity towards pathogenic microorganisms. This desirable characteristic makes copper oxides commercially applicable in paints, fabrics, agriculture and in hospitals either as constituent powders or as coated films.4,5 Numerous reports discussed the antibacterial activities of elemental Cu, CuO and Cu2O relating their particle size effect,6 morphology,7 dissolution of copper ions in different medium8 etc. CuO antibacterial action has been connected with a sudden decline in cell membrane integrity and production of reactive oxygen species (ROS).9 The redox cycling between Cu(I) and Cu(II) intend to generate superoxide species in contributing degradation of biomolecules. It is believed that in bacterial cells, Cu(II) ions are reduced by sulfhydryl to cuprous Cu(I) ions. These reduced ions are responsible for causing oxidative stress via Cu(I) – driven ROS.10 Prominent antibacterial potency of Cu2O over CuO has been noted in the literature. Release of ionic Cu from metallic Cu surfaces has also been suggested as a reason behind its antimicrobial effect.11 Leaching of copper ions from different copper salts including micron and nano sized copper oxides to cause bactericidal activity is another mechanism for copper oxides.12 Overall, Cu(I) and metallic Cu are shown to possess higher antimicrobial potency than the Cu(II) state.13 Despite these reports, the exact pathway of attacking microbes by copper oxides is not clearly remarked in the literature.Three important questions so far have remained open about the contact killing of bacteria by copper oxides. (i) How do metal oxides actively damage cells during contact killing? (ii) What is the toxicity mechanism of copper ions in contact killing? and (iii) whether cuprous (Cu+) and cupric (Cu2+) ions follow similar mechanism in contact killing?
Till now, the relevance of oxidation state to antibacterial activity of copper oxides is vaguely understood. The main objectives of this study are to impart a clear picture of the mode of antibacterial action of CuO and Cu2O with respect to oxidation state and the importance of the solid particles to mediate cellular uptake and further release of metal ions inside the cell. Comparisons were made with CuBr and CuCl2 salts to address the effect of metal ion toxicity leading to cell death. Enzyme assay results indicated that the intracellular proteins are directly damaged by Cu2O nanoparticles and cuprous salt. Experiments showed that CuO is also capable of damaging fumarase enzyme of bacteria, but significantly slower than Cu(I). This suggests Cu(I) compounds are chelated with such enzymes resulting into killing of bacterial cells.
Results
Preparation of tryptophan (Trp) capped CuO and Cu2O nanoparticles
For the synthesis of CuO, 0.1 mM copper nitrate and 0.2 mM sodium hydroxide were dissolved in 50 mL distilled water each and mixed together. The amino acid L-tryptophan, a capping agent of 0.03 mM (0.061 g) was then added into the solution. The mixture was heated at 80–90 °C for 2–3 hours under constant stirring. A black precipitate of Trp capped CuO was obtained and then purified. For Cu2O, the same procedure was followed; a reducing agent, 5 mL of 2 M hydrazine (N2H4) was added at a rate of 1 mL min−1 into the above stirring solution. The change in colour from blue to reddish brown was noted.For the synthesis of nano sized Cu2O crystals, mild reducing agent (hydrazine) and its concentration to metal ion was controlled. We had initially attempted with NaBH4 and NH2OH·HCl but these reducing agents resulted into the reduction of Cu2+ to Cu2O and Cu as well (see ESI Fig. S1†). The importance of employing hydrazine as a reducing agent, which produces N2 gas, is to maintain the reaction system in an inert atmosphere and prevent further oxidation of Cu2O to CuO. Care was taken so that the intermediate complex [Cu(OH)4]2− obtained would not result into CuO precipitation in the colloidal solution.
XRD & TEM
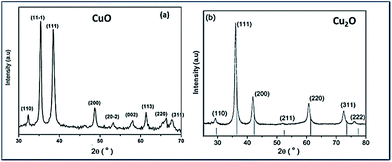
Fig. 1(a) and (b) show the XRD patterns of the Trp-capped CuO and Cu2O respectively. In both the diffractograms significant broadening could be observed, due to effective capping of tryptophan for controlling the size. The XRD reflections of CuO match that of JCPDS no. 48-1548 corresponding to monoclinic structure (Fig. 1(a)).Fig. 1(b) illustrates Trp-capped Cu2O nanoparticles. No diffraction lines associated with impurities were detected. JCPDS 05-0667 matches with cubic fcc structure of Cu2O representing 〈110〉, 〈111〉, 〈200〉, 〈220〉, 〈311〉 and 〈222〉 planes.
TEM images are shown in Fig. 2. It is quite evident from the Fig. 2a–d, that the particles are well separated and uniformly distributed. High morphological uniformity of these CuO (Fig. 2a and b) and Cu2O (Fig. 2c and d) crystals are evident from the TEM images.
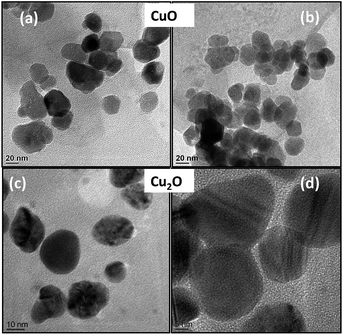 | ||
| Fig. 2 Transmission electron micrographs of tryptophan capped CuO (a), (b) and Cu2O nanoparticles (c), (d). | ||
Among the particles examined (Fig. 2a and b), more than 70% belonged to the size range of ∼30 nm and ∼40 nm for CuO and Cu2O respectively. Through continuous conversion of Cu (OH)2 to CuO (Fig. 2a) and well controlled reduction of N2H4 to Cu2O fully grown spherical particles were produced (Fig. 2c and d).
The susceptibility examination
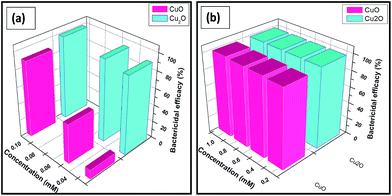
The antibacterial activities of both copper oxides are shown in Fig. 3. The ability of copper oxides to kill E.coli was confirmed based on the decrease in number of the colonies observed on the agar plates. The suspensions of various concentrations of Cu(I) and (II) oxides were incubated for 18 h and the bactericidal efficacies were monitored and the results were summarized in Table 1.| Concentration | Trp-CuO | Trp-Cu2O |
|---|---|---|
| Half MIC (IC50) | 0.025 mM | 0.02 mM |
| MIC (IC100) | 0.1 mM | 0.05 mM |
| MBC | 0.25 mM | 0.1 mM |
The results are shown in Fig. 3(a) and (b) in the lowest concentration range (0.025–0.1 mM) and the highest concentration range (0.25–1 mM), respectively. Cu2O NPs exhibited the MIC values of lowest IC50 equal to 0.02 mM and IC100 higher than 0.05 mM. CuO NPs also depicted sensitivity against E.coli almost comparable with IC50 value equal to 0.025 mM and IC100 to 0.1 mM. In contrast, the growth of bacterial strains tested for tryptophan (0.03 mM) and 5% inhibition was noted with concentrations higher than 0.03 mM.
It is worthy saying that up to 97% of microorganisms (E.coli) were killed in the presence of Cu2O nanoparticles at the concentration of 0.05 mM whereas for CuO nanoparticles at 0.05 mM only 73% bactericidal activity were observed.
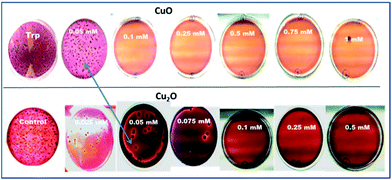
Images of the colonies observed on the plates at different concentrations of CuO and Cu2O are shown in Fig. 4.
From the Fig. 4, the minimum inhibitory concentrations (MIC) for CuO and Cu2O were found as 0.1 mM and 0.05 mM respectively. The minimum bactericidal concentrations (>98% inhibition) were noted as 0.1 mM and 0.25 mM for Cu2O and CuO respectively.
Cell integrity study
The bacterial membrane serves as a structural component which may become compromised during a biocidal challenge such as exposure to antibiotics, biocides etc. Therefore, release of intracellular components is a good indicator of membrane integrity.14 Small ions such as potassium and phosphate tend to leach out first, followed by molecules such as DNA, RNA and other materials. Since these nucleotides have strong absorption at 260 nm, they are termed as “260 nm absorbing materials”.15The UV-visible study on the release of 260 nm absorbing materials upon addition of copper oxide nanoparticles of MBC concentration to an E.coli suspension is shown in Fig. 5. The OD of the bacteria suspension is quickly elevated upon the addition of copper oxides to 20% and 35% for CuO and Cu2O respectively in 15 min. The ratio did not increase significantly since then. This quick release of 260 nm absorbing materials is in good agreement with fast killing kinetics of the Cu2O nanoparticles.
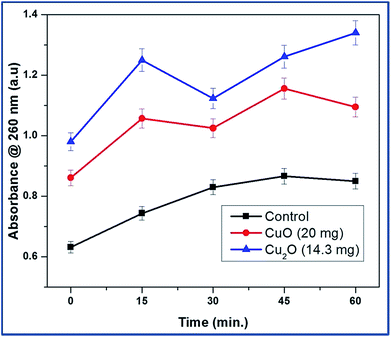 | ||
| Fig. 5 Release of 260 nm absorbing materials from E.coli upon treatment with CuO and Cu2O nanoparticles at typical MBC concentration. | ||
It was found that proteins were released into the surrounding medium on account of the loss of membrane integrity induced by the nanoparticles. It is evident from the results of the Trp-CuO and Trp-Cu2O nanoparticles mediated cultures showed a sudden increase in the absorbance in first 15 min. It can also be seen from the graph that Trp-Cu2O nanoparticles have more impact on the membrane integrity of the cell than the Trp-CuO nanoparticles. This is the indicator for us whether the oxidation state would play a role towards antibacterial activity. However, at longer time, the OD value inclined to decrease with time. This decrease in OD might be due to the precipitation caused by phosphonated entities within the cytoplasm such as adenosine triphosphate or nucleic acids.16 The copper oxides collected with heterogeneous cell components of E.coli were statistically higher than those in the control group.
The above study was successful in proving that the nanoparticles have caused membrane damage. The cell membrane is known to have pores that are of nanometer size. The nanoparticles having appropriate charge and size can cross the membrane and cause cell death either by the production of reactive oxygen species or by the disruption of cell function thereby affecting proteins and DNA.17
Measurement of superoxide anion production using NBT-light system
The cells harvested after incubation with copper oxide nanoparticles of MBC concentrations (0.1 mM and 0.25 mM for CuO and Cu2O respectively) were washed twice with saline solution.The supernatant fraction was used to measure superoxide anion activity by the method of Rae et al.18 with slight modification. Superoxide anion radicals are formed by the addition of one electron to molecular oxygen.19 This is considered as the “primary” ROS and can interact with other molecules to form “secondary” ROS. The nanoparticles were tested for the production of superoxides by measuring the reduction of NBT to blue formazan. The assay involves the ability of SOD to inhibit the reduction of nitrobluetetrazolium (NBT) by superoxides, which is generated by the reaction of photo reduced riboflavin and oxygen.
The Fig. 6 explains the measure of superoxide anion production from copper oxides and compared with standard riboflavin. In the case of CuO, the O2−˙ level decreased significantly indicating that direct consumption of O2−˙ for the reaction with CuO to form Cu(I). The diffusion controlled reactions are more feasible outside the cells in the presence of CuO.20
| Cu(II) + O2−˙ → Cu(I) + O2 |
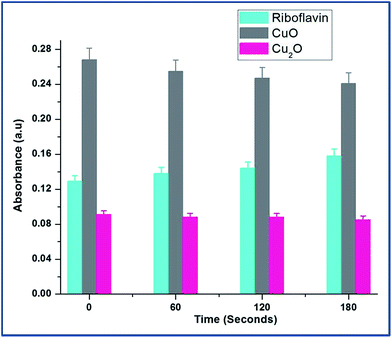 | ||
| Fig. 6 Dynamics of superoxide anion production was probed by riboflavin assay throughout the growth of E.coli in the presence of CuO and Cu2O nanoparticles at 0.25 mM and 0.1 mM concentrations respectively. The data points are the average of three repeated experiments. For tryptophan and riboflavin standard results, see ESI Table S4.† | ||
At the same time, Cu2O did not produce any superoxide radicals. It can be explained that Cu2+ ion rapidly reacts with superoxide making it to participate in redox cycling; therefore leading to sustained oxidative stress.
Determination of OH−˙
Hydroxyl radical (OH−) is the most reactive oxygen radical known, and reacts very quickly with almost every type of molecule found in the living cells.21 Such reactions will probably facilitate the combination of two OH radicals to form hydrogen peroxide (H2O2). The production of hydroxyl ion by the nanoparticle was tested based on the reduction of deoxyribose by the hydroxyl ions. The absorbance of several concentrations of Trp-CuO and Trp-Cu2O used to calculate the concentrations of hydroxyl ions produced. The results revealed that the copper oxide nanoparticles were successful in generating hydroxyl ions via the catalytic conversion of intracellular H2O2.From Fig. 7, it is evident, at relatively lower concentrations (0.05 and 0.075 mM), the levels of OH−˙ are higher for CuO than Cu2O. The Cu(II) ions are reduced by superoxide species to cuprous state and in turn produce H2O2. These intracellularly generated H2O2 converted to OH−˙ via a Fenton like reaction.
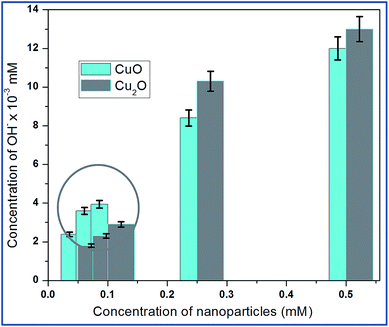 | ||
| Fig. 7 Intracellular production of OH˙ from the suspensions of different concentrations of copper oxides. The circled area shows that CuO at lower concentration produce large quantities of OH radicals. The hydroxyl radical's quantification is calculated based on the standard H2O2 of different samples (Fig. S5†). | ||
The increase in concentration of OH˙ radicals is high for CuO even at lower dose levels (<0.1 mM). At 0.5 mM the OH radical concentration is less than Cu2O. These results indicate that free radicals such as OH˙ radicals are generated from the redox cycling of intracellular copper catalyzed by periplasmic Cu–Zn super oxide dismutase (SOD1) found in E.coli so called the defence mechanism of E.coli.22
Discussion
The mechanism by which copper kills the cells has been elusive. Copper, a redox active transition metal could labialize between two redox states oxidized cupric and reduced cuprous species.23 The first indication while testing for antibacterial activity of cupric and cuprous oxides is the absence of superoxide species from Cu2O. The main question rose here, why did Cu2O not render super oxide(s)? Working towards this key issue, interesting results were found. A long-standing hypothesis is that copper reacts with endogenous H2O2 to generate hydroxyl radicals in a process analogous to the Fenton reaction to generate O2−˙ or hydroxyl radicals (OH˙).24 This supports our results, (Fig. 6), no super oxide species generated from Cu2O, but higher OH˙ radicals (Fig. 7) through Fenton reaction.It is quite evident from the present work, bacteriostatic action of CuO nanoparticles mainly originates from the direct interaction of CuII species with the cell components. According to the Fenton reaction, CuII is reduced to CuI by the cell components and is majorly responsible for the inactivation of E.coli.25 The ROS production from metal oxides generally originates from the electron – donating nature of these ceramics. Nevertheless, it is believed, that the significant quantity of ROS (likely superoxide anions) must be rendered directly from the surface defect sites in nanocrystalline CuO.26
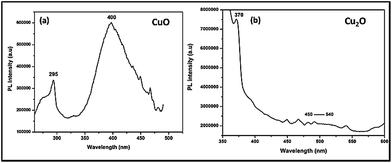
In order to confirm the above statement, Photo Luminescence (PL) spectra had been taken for Trp-capped copper oxides after incubation with microbes (Fig. 8). Surprisingly, CuO still preserved the surface defects at 400–450 nm, while Cu2O depicted completely the quenched emission at 450–500 nm. It must be pointed out here; tryptophan has been used as a capping agent for both the oxides, surface modification of metal oxides led to quenching of a defect related green emissions27 only with Cu2O not for CuO.
Then, what could be the possible mechanism for Cu2O? Park et al. demonstrated the direct evidence of Cu(I) chelated complex of dimethyl 1,10 phenonthraloine (DMP) which completely inactivated E.coli. Under anaerobic conditions, conversion of cupric to cuprous ions is the most feasible reaction and the latter was much more toxic to E.coli than the previous one.28
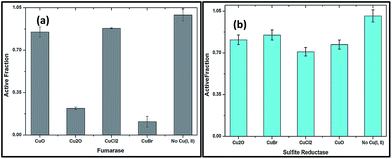
There are reports that the ability of cuprous state of copper directly damages certain enzymes of metabolites.29 In order to demonstrate the binding nature of Cu(I) with proteins, purified fumarase A (0.1 μM) as an iron cluster containing enzyme and non dehydratase enzyme sulphite reductase (0.1 μM) were employed. The copper oxides (I and II) and copper salts CuBr and CuCl2 of 100 μM were mixed independently to the medium containing enzymes for 3 min; then active fractions were tested for fumarase30 and sulphite reductase31 activities.
The rapid inactivation of fumarase A by copper(I) salts indicate the enzyme is directly damaged (Fig. 9a). The improved solubility of CuBr might inactivate fumarase A quickly. Low micromolar concentrations of Cu2O and CuBr directly damage the enzyme fumarase A. At the same time, CuO and CuCl2 were also capable of damaging fumarase, but it is significantly slower than Cu(I) salts. These results indicated that the poisoning of the enzymes by copper(I) occurred through a non-oxidative mechanism in an anaerobic buffer.
Fig. 9b shows that enzymes whose clusters are largely occluded by polypeptide are unlikely to be targets of copper. Indeed, metabolic pathways that include non-dehydratase cluster enzymes—such as sulfite reductase remained functional when cells were exposed to copper oxides and salts, as evidenced by growth in medium. These results proved that Cu(I) might chelate proteins so that it fails to associate with DNA and/or undergo cycles of oxidation and reduction.
The biocidal action of Cu2O must be based on the binding nature of Cu(I) ions with thiol groups, just like Ag(I).32 Ascorbic acid, glutathione (GSH) and other amino acids from intracellular proteins are ready to chelate Cu(I) ions and tend to reduce cupric ions to cuprous33 (by any means) and then bonding the latter. This was strongly indicating that Cu2O binds with intracellular proteins. In Gram negative bacteria such as E.coli, the smallest water soluble molecules (including antibiotics) enter the intracellular membrane by diffusion, through the channels of non-specific porin located in the outer membrane.34 It helps in up-taking basic amino acids including tryptophan. So, the observed enhanced antibacterial action is possible for copper(I) oxide through, binding of protein components to Cu(I) but not through ROS/oxidative stress and the ROS pathway is more applicable for CuO.
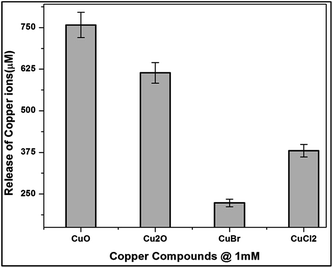
Another important factor to be addressed is the leaching of copper ions in the culture medium. Two aspects have to be looked at with respect to the intracellular bioavailability, namely the internalization of particles and the increase in water soluble copper ions within the cell.35 Though copper is an essential trace element, elevated intracellular levels may exceed copper homeostasis, giving rise to pro-oxidative reactions.
E.coli cells were incubated with copper compounds for 24 h to quantify the bioavailability and intracellular distribution of ionic copper (Fig. 10). The cells were lysed and the soluble cytoplasmic fractions were isolated. The primary copper level of E.coli cells was found to be 22 μM in the cytoplasmic fraction. Treatment with copper compounds provoked a concentration-dependent copper accumulation in the cytoplasmic fraction.
Thus, the highest (MBC concentration) incubation concentration of 1 mM of copper compounds increased the primary cytoplasmic level of CuO, Cu2O, CuBr and CuCl2 to around 750 μM, 610 μM, 220 μM and 380 μM respectively.
The considerable amount of copper ions release from the nutrient media indicate that the media chloride ions interact with the oxide layers of copper oxides. The above results are in good agreement with Applerot et al.36 They stated from their results that the inactivation of E.coli by CuO is from intracellular ROS generation. Earlier reports stated that copper bacterial toxicity occur when the copper concentration to be 20 mg L.37 But the released copper ions were not enough to be toxic to the bacteria, might be considered negligible, whereas ROS generation and binding of proteins are the contributing factors of CuO and Cu2O respectively.
Conclusions
Taken together, this current work clearly indicates that the antimicrobial effect of copper oxides (I and II) differs by the oxidation state. Two different pathways are followed by these copper oxides, which are specific and independent. The percentage of reduction of viable cells was found to be high for cuprous oxide. The biocidal effect of CuO nanoparticles suggested the involvement of reactive oxygen species. Intracellular proteins have high affinity towards Cu2O rather than CuO was noticed. Significant inactivation of fumarase enzyme by Cu2O was demonstrated through binding of Cu(I) to the protein surface contributing the vital role.Acknowledgements
The authors thank VIT management for the constant support and encouragement. Ms Devi Meenkshi is greatly acknowledged for correcting the manuscript. Authors thank the reviewers for their useful comments.References
- O. Akhavan, R. Azimirad, S. Safad and E. Hasani, CuO/Cu(OH)2 hierarchical nanostructures as bactericidal photocatalysts, J. Mater. Chem., 2011, 21, 9634–9640 RSC.
- A. Kumar, P. K. Vemula, P. M. Aijayan and G. John, Silver-nanoparticle-embedded antimicrobial paints based on vegetable oil, Nat. Mater., 2008, 7, 236–241 CrossRef CAS PubMed.
- H. H. A. Dollwet and J. R. J. Sorensenson, Trace Elem. Med., 2001, 2, 80–85 Search PubMed.
- G. Borkow and J. Gabbay, Putting copper into action, copper-impregnated products with potent biocidal activities, FASEB J., 2004, 18, 1728–1730 CAS.
- J. J. Cooney and R. J. Tang, Quantifying effects of antifouling paints on microbial biofilm formation, Methods Enzymol., 1999, 310, 637–644 CAS.
- G. McDonnell and A. D. Russell, Antiseptics and Disinfectants, Activity, Action, and Resistance, Clin. Microbiol. Rev., 1999, 12, 147–179 CAS.
- Q. Lu, H. Pang and F. Gao, Morphology effect on antibacterial activity of cuprous oxide, Chem. Commun., 2009, 1076–1078 Search PubMed.
- G. Ren, D. Hu, W. C. Cheng, M. A. Vargas-Reus, P. Reip and R. P. Allaker, Characterization of copper oxide nanoparticles for antimicrobial applications, Int. J. Antimicrob. Agents, 2009, 33, 587–590 CrossRef CAS PubMed.
- S. Jadhav, S. Gaikwad, M. Nimse and A. Rajbhoj, Copper oxide nanoparticles, synthesis, characterization and their antibacterial activity, J. Cluster Sci., 2011, 22, 121–129 CrossRef CAS.
- W. Wang, J. Ren, S. Sun, L. Zhang, L. Wang and J. Chang, Crystallography Facet-Dependent Antibacterial Activity, The Case of Cu2O, Ind. Eng. Chem. Res., 2011, 50, 10366–10369 CrossRef.
- R. Amal, C. Lee, C. Gunawan, W. Yang Teoh and C. P. Marquis, Cytotoxic origin of copper (II) oxide nanoparticles, Comparative studies with micron-sized particles, leachate, metal salts, ACS Nano, 2011, 5, 7214–7225 CrossRef PubMed.
- K. Midander, P. Cronholm, H. L. Karlsson, K. Elihn, K. Möller, C. Leygraf and I. O. Wallinder, Surface characteristics, copper release, and toxicity of nano- and micrometer-sized copper and copper (II) oxide particles, A cross-disciplinary study, Small, 2009, 5, 389–399 CrossRef CAS PubMed.
- J. I. Nieto-Juarez, K. Pierzchla, A. Sienkiewicz and T. Kohn, Inactivation of MS2 coliphage in Fenton and Fenton-like systems, Role of transition metals, hydrogen peroxide and sunlight, Environ. Sci. Technol., 2010, 44, 3351–3356 CrossRef CAS PubMed.
- C. E. Santo, N. Taudte, D. H. Nies and G. Grass, Contribution of copper ion resistance to survival of Escherichia coli on metallic copper surfaces, Appl. Environ. Microbiol., 2008, 74, 977–986 CrossRef CAS PubMed.
- L. M. Gaetke and C. K. Chow, Copper toxicity, oxidative stress, and antioxidant nutrients, Toxicology, 2003, 189, 147–163 CrossRef CAS.
- T. Ikeda, A. Ledwith, C. H. Bamford and R. A. Hann, Interaction of a polymeric biguanide biocide with phospholipsid membranes, Biochim. Biophys. Acta, 1984, 769, 57–66 CrossRef CAS.
- C. Z. Chen and S. L. Cooper, Interactions between dendrimer biocides and bacterial membranes, Biomaterials, 2002, 23, 3359–3368 CrossRef CAS.
- T. D. Rae, P. J. Schmidt, R. A. Pufahl and T. V. O'Halloran, Undetectable intracellular free copper, The requirement of a copper chaperone for superoxide dismutase, Science, 1999, 284, 805–808 CrossRef CAS.
- L. Macomber, C. Rensing and J. A. Imlay, Intracellular copper does not catalyze the formation of oxidative DNA damage in Escherichia coli, J. Bacteriol., 2007, 189, 1616–1626 CrossRef CAS PubMed.
- W. Hugo and G. Snow, Biochemistry of antibacterial action, Chapman & Hall, London, 1981 Search PubMed.
- A. Ivask, O. Bondarenko, N. Jepihhina and A. Kahru, Profiling of the reactive oxygen species-related Eco toxicity of CuO, ZnO, TiO2, silver and fullerene nanoparticles using a set of recombinant luminescent Escherichia coli strains, Differentiating the impact of particles and solubilized metals, Anal. Bioanal. Chem., 2010, 398, 701–716 CrossRef CAS PubMed.
- W. X. Fan, H. Wang, M. Cui, D. Zhang, Y. Zhang, T. Yu and L. Guo, Differential oxidative stress of octahedral and cubic Cu2O micro/nanocrystals to Daphnia magn, Environ. Sci. Technol., 2012, 46, 10255–10262 CAS.
- J. E. J. Weckx and H. M. M. Clijsters, Oxidative damage and defense mechanisms in primary leaves of Phaseolus vulgaris as a result of root assimilation of toxic amounts of copper, Physiol. Plant., 1996, 96, 506–512 CrossRef CAS PubMed.
- D. R. Lloyd and D. H. Phillips, Oxidative DNA damage mediated by copper(II), iron(II) and nickel(II) fenton reactions, Evidence for site-specific mechanisms in the formation of double-strand breaks, 8-hydroxydeoxyguanosine and putative intra strand cross-links, Mutat. Res., 1999, 424, 23–36 CrossRef CAS.
- A. Battistoni, F. Pacello, S. Folcarelli, M. Ajello, G. Donnarumma, R. Greco, M. G. Ammendolia, D. Touati, G. Rotilio and P. Valenti, Increased expression of periplasmic Cu, Zn superoxide dismutase enhances survival of Escherichia coli invasive strains within nonphagocytic cells, Infect. Immun., 2000, 68, 30–37 CrossRef CAS PubMed.
- C. G. Rensing and G. Grass, Escherichia coli mechanisms of copper homeostasis in a changing environment, FEMS Microbiol. Rev., 2003, 27, 197–213 CrossRef CAS.
- E. Cabiscol, J. Tamarit and J. Ros, Oxidative stress in bacteria and protein damage by reactive oxygen species, Int. Microbiol, 2000, 3, 3.3–3.8 Search PubMed.
- H. J. Park, T. M. Thuy Nguyen and J. Yoon, Role of reactive oxygen species in Escherichia coli inactivation by cupric Ion, Environ. Sci. Technol., 2012, 46, 11299–11304 CrossRef CAS PubMed.
- M. Cho, J. Kim, J. Y. Kim, J. Yoon and J.-H. Kim, Mechanisms of Escherichia coli inactivation by several disinfectants, Water Res., 2010, 44, 3410–3418 CrossRef CAS PubMed.
- V. Massey, Fumarase, Methods Enzymol., 1955, 1, 729–735 CAS.
- K. R. Messner and J. A. Imlay, The identification of primary sites of superoxide and hydrogen peroxide formation in the aerobic respiratory chain and sulfite reductase complex of Escherichia coli, J. Biol. Chem., 1999, 274, 10119–10128 CrossRef CAS PubMed.
- Y. Gong, T. Andelman, G. F. Neumark, S. O'Brien and I. L. Kuskovsky, Origin of defect-related green emission from ZnO nanoparticles, Effect of surface modification, Nanoscale Res. Lett., 2007, 2, 297–302 CrossRef CAS.
- A. Semisch, J. Ohle, B. Witt and A. Hartwig, Cytotoxicity and genotoxicity of nano – and microparticulate copper oxide: role of solubility and intracellular bioavailability, Part. Fibre Toxicol., 2014, 11, 1–16 CrossRef PubMed.
- S. Y. Liau, D. C. Read, W. J. Pugh, J. R. Furr and A. D. Russell, Interaction of silver nitrate with readily identifiable groups, Relationship to the antibacterial action of silver ions, Lett. Appl. Microbiol., 1997, 25, 279–283 CAS.
- A. C. F. Gorren, A. Schrammel, K. Schmidt and B. Mayr, Decomposition of S-nitrosoglutathione in the presence of copper ions and glutathione, Arch. Biochem. Biophys., 1996, 330, 219–228 CrossRef CAS.
- G. Applerot, J. Lellouche, A. Lipovsky, Y. Nitzan, R. Lubart, A. Gedanken and E. Banin, Understanding the antibacterial mechanism of CuO nanoparticles, revealing the route of induced oxidative stress, Small, 2009, 5, 389–399 CrossRef PubMed.
- Z. Wang, Y. H. Lee, B. Wu, A. Horst, Y. Kang, Y. J. Tang and D. R. Chen, Anti-microbial activities of aerosolized transition metal oxide nanoparticles, Chemosphere, 2010, 80, 525–529 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed methods of preparation and characterization are given. Inactivation of bacterial cells at various concentrations of copper oxides; standard measurements of riboflavin and H2O2 and IR spectral details of copper oxides are available as ESI. See DOI: 10.1039/c4ra12163e |
| ‡ Equally contributed. |
| This journal is © The Royal Society of Chemistry 2015 |