Synthesis of high purity Cr2AlC nanolamellas with improved tribological properties for oil-based additives
Maoquan Xueab,
Xianghua Zhanga,
Hua Tanga and
Changsheng Li*a
aSchool of Materials Science and Engineering, Jiangsu University, Zhenjiang, Jiangsu Province 212013, P.R.China. E-mail: changshengli2008@163.com; Fax: +86 511 8879 0268; Tel: +86 511 8879 0268
bChangzhou Institute of Light Industry Technology, Changzhou, Jiangsu Province 213164, P.R.China
First published on 1st August 2014
Abstract
Herein, a novel simple method is presented to synthesize highly pure Cr2AlC powder by heating a 2Cr/xAl/C (molar ratio, x = 1, 1.1, 1.2) powder system between 1300 °C and 1400 °C with preliminary magnetic stirring mixing in alcohol. The purity of Cr2AlC is sensitive to the final temperature and raw material scale, and the excess Al plays a distinct role in improving the purity of the Cr2AlC. The tribological properties of Cr2AlC as an additive in 100SN base oil were evaluated by a UMT-2 ball-on-disc friction and wear tester. The results show that under determinate conditions, the base oil containing 0.6 wt% Cr2AlC sample presented good tribology performance under a load of 10 N. The improved tribological properties of the Cr2AlC samples could be attributed to the formation of a tribofilm in the friction process.
1. Introduction
Chromium aluminum carbides, Cr2AlC, belong to a special group of the materials known as layered ternary compounds. This new class of materials features a hexagonal crystalline structure and can be represented by a general formula of Mn+1AXn (MAX), where n = 1, 2, or 3, M is an early transition metal, A is an A-group element, and X is carbon or nitrogen,1–7 the unique combination of ceramic and metallic properties has attracted considerable interest. Among them, Cr2AlC, is a member of the novel 211 ternary compounds exhibits outstanding ceramic properties such as a low density,8 high melting point and thermal stability,9 a low thermal expansion coefficient,10,11 high strength at high temperatures,12 and excellent oxidation resistance.13,14 Meanwhile, Cr2AlC possesses metallic properties including relatively high electrical and thermal conductivities,15,16 well resistant to thermal shock,4 good damage tolerance,17,18 and easy machinability.To date, several methods including mechanically activated hot pressing,19 hot isostatic pressing,20 hot pressing,13,21 spark plasma sintering,22 and so on have been adopted to synthesize Cr2AlC from different mixtures with different mole ratios. These synthesis processes, however, usually require high energy ball milling and certain sintering equipment requirements, which lead to raw material easy being oxidation, energy and time consuming, complicated productive process and low production efficiency.23–27 Therefore, it is still a great challenge to develop a facile and effective process to fabricate Cr2AlC with high purity. In addition, it was found that Cr2AlC has an excellent tribological property, especially at elevated temperatures.28–30 The relatively low coefficient and wear rate are attributed to the amorphous or nanocrystalline tribofilms form on both contact surfaces. However, to the best of our knowledge, little work focused on the tribological properties of Cr2AlC as lubrication additive.
In this study, laminate-like Cr2AlC crystals with high purity were synthesised by pressureless sintering raw powders at 1300–1400 °C in a flowing argon atmosphere, the raw powders were directly mixed by magnetic stirring in alcohol. The tribological properties of Cr2AlC samples as additives in the 100SN base oil were also investigated.
2. Experimental
2.1 Synthesis of laminated Cr2AlC
Cr (99.0% pure, −200 mesh), Al (99.0% pure, −200 mesh), and graphite (99% pure, ∼5 μm) (all from Sinopharm Chemical Reagent Co. Ltd., Shanghai China) powders were used in this work. About 5 g raw powders with stoichiometric molar ratio of 2Cr/xAl/C (x = 1, 1.1, 1.2) were mixed by magnetic stirring in absolute alcohol at 70 °C. Alcohol evaporated in heating process, ∼1 h, alcohol evaporated completely. After being dried and sieved with 200-mesh screen, the blended powder was placed in a stainless steel mould with diameter of 25 mm, the pressure was 30 MPa. Then, the cold pressing slices were loaded into corundum crucible and sintered in tube furnace. The samples were heated at a rate of 5 °C min−1 in flowing argon atmosphere until the final temperatures (from 1300 to 1400 °C) were reached, at which the sintering time was 30 min. Finally samples were crushed and grinded into powder.2.2 Characterisation of Cr2AlC samples
The raw blended powder was analyzed with differential scanning calorimetry (DSC) in an instruments analyzer (NETZSCH-Ger tebau GmbH Selb/Germany). The runs were performed in an argon atmosphere, with a 10 °C min−1 temperature increase rate from room temperature up to 1300 °C. The phases of prepared Cr2AlC ceramics powders were analyzed using a D8ADVANCE diffractometer and Cu Kα radiation in the 2θ range between 10°and 80°, operating at 40 kV and 20 mA, λ = 0.1546 nm, respectively, data analysis with Jade software. The morphologies and microstructures of Cr2AlC ceramics were determined by Scanning Electron Microscopy (SEM) (JEOL JXA-840A).2.3 Tribological properties of laminated Cr2AlC crystals as lubrication additive
Different mass fractions of the as-prepared Cr2AlC powder from 2Cr/1.2Al/C sintered at 1400 °C were dispersed in 100SN base oil via 2 h ultrasonication without any active reagent, and then a series of suspended oil samples were obtained. The tribological properties of the base oil containing Cr2AlC were evaluated on a UMT-2 ball-on-disc friction and wear tester. The testing of friction reduction and wear resistance was conducted at rotating speeds of 5 m min−1, and loads of 10–30 N for sliding distance 200 m. The material of the upper sample is a 440C stainless steel ball with a diameter of 10 mm, hardness of 62 HRC and the counterpart is a 45 steel disc of Φ 25 mm × 5 mm in size. The friction coefficient was recorded automatically with a strain gauge equipped with the tester. The wear scars widths were measured by a common optical microscope. Morphologies of friction surfaces were examined using a JSM-5600LV scanning electron microscope (SEM). The elements of the friction surface were analyzed using energy-dispersive X-ray spectroscopy (EDS).3. Results and discussion
3.1 Phase analysis of Cr2AlC
Fig. 1 shows the XRD patterns for blended powders after magnetic stirred in absolute alcohol, the upper right inset shows the SEM. The main phases of the powders included graphite, aluminum and chrome elementary substance, the blended powders is small sheet with 10 μm.Fig. 2 shows typical XRD patterns of as-synthesized products obtained from 2Cr/xAl/C (x = 1, 1.1, 1.2) powders mixtures after sintered at various temperatures of 1300–1400 °C. It is found that all the samples are contented Cr2AlC phase, the (103) main peak of Cr2AlC at 42.1° is obvious. When the powder ratio is 2Cr/1Al/1C (as shown in Fig. 2(a)), for the specimen synthesized at 1300 °C, Cr2AlC was found to be main crystalline phases, Cr2Al and Cr7C3 were presented as minor phase. As the sintering temperature was increased to 1350 °C, Cr2Al phase was gradually decreased while the contents of Cr7C3 and Cr2AlC phases were increased. With further increasing the sintering temperature to 1400 °C, the Cr2Al and Cr7C3 second phases were disappeared, major phases were identified as Cr2AlC. For the specimen synthesized from 2Cr/1.1Al/1C system shown in Fig. 2(b), most of the phases synthesized at the temperatures ranged from 1300 to 1400 °C were similar with those of synthesized from 2Cr/1Al/1C system, however, contents of Cr2Al and Cr7C3 phases were both decreased while the intensity of Cr2AlC peaks are getting stronger. As shown in Fig. 2(c), with the further addition of Al into the raw materials, that is 2Cr/1.2Al/1C system, Cr2Al and Cr7C3 second phases were further decreased, even disappeared at the sintering temperature 1300 and 1350 °C, so the Cr2AlC phase was gradually increased, with the specimen synthesized temperature high to 1400 °C, the second phases were disappeared, all phases were identified as Cr2AlC.
 | ||
| Fig. 2 XRD patterns of 2Cr/xAl/C powders after sintered at different temperature with (a) x = 1, (b) x = 1.1 and (c) x = 1.2. | ||
As shown in Fig. 2, specimen synthesized by pressureless sintering method using Cr, Al and graphite mixed powder as a raw materials at the temperature range of 1300–1400 °C, Cr2AlC main crystalline phase with small amount of Cr2Al and Cr7C3 were identified, also the contents of Cr2Al and Cr7C3 second phases were gradually decreased while the intensity of Cr2AlC peaks are getting stronger with sintering temperature increased. For the specimen synthesized at 1400 °C, high purity Cr2AlC phase can be synthesized.
Fig. 3 shows XRD patterns of the specimen synthesized using the Cr, graphite and different content Al powder mixture by a pressureless sintering at 1400 °C. With the addition of excessive Al into the raw materials, the relative peak intensity of Cr2AlC obviously increased, which demonstrated that the introduction of excessive Al increased the purity of Cr2AlC in the products.
3.2 Microstructure observation of samples by SEM
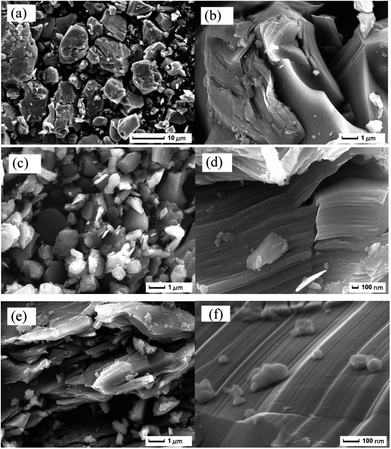
Fig. 4 shows the SEM images of the synthesized Cr2AlC powders obtained at 1400 °C. Fig. 4(a) is the micrograph of the sample sintered from powder of 2Cr/1Al/1C, as can be seen from the image, the obtained samples were irregular particles stacked by laminated layers with average size of less than 5 μm. Fig. 4(b) is enlarged micrograph of Fig. 4(a), indicating that the irregular particles are composed of nanoplates with average thickness in the range of 20–30 nm and further confirming the layered nature of the material. Fig. 4(c) and (d) shows the SEM images of the sample sintered from powder of 2Cr/1.1Al/1C. As shown in Fig. 4(c), the sample was composed of a lot of plate-like and block-shaped particles, these particles with different size and smooth surface, further observation shows that the particles have melting imprint, indicating the formation of liquid phase at high temperature. Fig. 4(d) is enlarged SEM image of fractured particles, laminated-like structure of Cr2AlC is obvious stacked by many uniform nano slices with thickness of about 100 nm, rupture and convolution feature was presented. Fig. 4(e) is SEM images of the sample sintered from powder of 2Cr/1.2Al/1C, in which the particles of this powder are larger, thickness is generally about 50 nm. Fig. 4(f) is enlarged SEM image of Fig. 4(e), the growth pattern of laminated-like structure is obviously.3.3 Formation process of Cr2AlC
In order to understand the formation process of Cr2AlC, the phase of the sample sintered at different temperatures from 700 °C to 1400 °C using mixed powder of 2Cr/1.2Al/C as starting materials were investigated by XRD technique.Fig. 5 shows XRD patterns of the powders synthesized under different temperatures. According to Fig. 5, C and Cr peaks can be clearly seen in the diffraction profile at 700 °C, peaks at 2θ = 40 to 44° appeared broadening that originated from the formation of Cr–Al phases when sintered at 700 °C. For the sample heated to 850 °C and 1050 °C, it can be seen that except for un-reacted C and Cr phases, Cr2AlC phase has been formed and Cr5Al8, Cr2Al peaks also can be observed as intermediate phases. With increasing temperature to 1200 °C, C at 2θ = 26.6° and Cr5Al8 at 2θ = 24.1° peaks abruptly reduced, Cr2Al, Cr7C3 and Cr2AlC were detected. Except main crystalline phase Cr2AlC, only quite weak Cr2Al and Cr7C3 peak were detected in the sample sintered at 1300 °C. When the temperature was 1350 °C, main crystalline phase Cr2AlC and a few Cr7C3 were detected. Further more, when the sintering temperature was as high as 1400 °C, only single-phase Cr2AlC was detected in the sample. The results indicated that the highly pure Cr2AlC powder seemed to be easily synthesized by using liquid magnetic stirring and pressureless sintering process from 2Cr/1.2Al/1C powder mixtures.
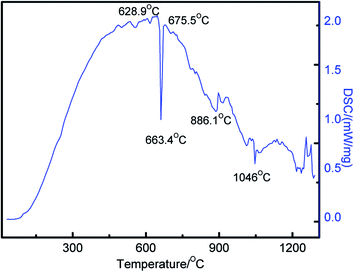
DSC survey was conducted to investigate the formation of products during the sintering process. Typical DSC curve for the blended powders of 2Cr/1.2Al/1C system at a heating rate of 10 °C min−1 is shown in Fig. 6. It can be seen that there is an obvious endothermic peak at 663.4 °C, and there are a lot of endothermic and exothermic peaks at the temperatures range from 886.1 to 1300 °C, it is sure that the peaks correspond to the frequent reaction and form new compounds. Based on the binary phase diagram of the Cr–Al system,31 it can be presumed that aluminum melted at 663.4 °C, and reacted with Cr particles to form CrxAly intermetallics. These endothermic and exothermic peaks at temperatures from 886.1 to 1046.6 °C correspond to the reaction of forming Cr5Al8 and Cr Cr2Al. It is considered that the endothermic and exothermic peaks at higher temperatures resulted from the reactions of formation Cr2AlC and Cr7C3 by expense of Cr5Al8, Cr2Al and graphite gradually.
Based on the previous work of Cr2AlC powder synthesis and the results of this study, the synthesis mechanism of pressureless sintering Cr2AlC powder was presented. Fig. 7 shows the schematic diagram of the synthesized samples obtained by the pressureless sintering process. At the first stage, Al easily melted at 663.4 °C due to its low melting point, and diffusion in the pore of samples, formation molten pool, chromium and graphite was wrapped in the liquid phase of Al. With the sintering temperature increased, chromium and aluminum begins to react in the contact interface, promote formation of chrome aluminum intermetallic. When the sintering temperature increased to 850 °C, the formation of the intermediate phase mainly for the Cr5Al8 and a small amount of Cr2Al, at the same time have a small amount of Cr2AlC, mainly reaction formation by Cr5Al8, chromium and graphite, also unreacted Cr and graphite are detected. At a higher temperature of 1050 °C, Cr5Al8 reaction with the raw material of chromium, aluminum to form Cr2Al, and at the same time Cr5Al8, Cr2Al react with graphite to form Cr2AlC, leading to the reaction product of Cr5Al8 content decreased, Cr2Al content increased, chromium and graphite continues to drop, Cr2AlC continued to rise. When the sintering temperature continues to rise to 1200 °C above, the spawning of Cr2Al reacted with graphite to form Cr2AlC, part of chromium reacted with graphite to form Cr7C3, as the sintering temperature increased to 1400 °C, the high purity Cr2AlC is finally fabricated.
3.4 Friction and wear properties of laminated Cr2AlC crystals
The tribological behaviors of the as-prepared Cr2AlC powders as lubrication additive in 100SN base oil were investigated by a UMT-2 ball-on-disc friction and wear tester. Fig. 8(a) shows the friction coefficients vs. sliding distance curves of base oil at 10 N load under 5 m min−1 sliding speed with different Cr2AlC concentrations (0–5 wt%). It can be observed that the friction coefficient is sensitive to the additive concentration of the laminated Cr2AlC particles. The friction coefficient of the lubricating system is obviously decreased by adding synthesized laminated Cr2AlC over a wide concentration range of 0.6–3 wt%, the friction coefficients decreased slightly to a steady value with the sliding distance. When the concentration of synthesized laminated Cr2AlC is 0.6 wt%, the best friction coefficient-reducing property is obtained. Contrary to the lower concentrations, the base oil with 5 wt% synthesized laminated Cr2AlC has a relatively higher friction coefficient compared with the base oil. This can be attributed to the fact that the dispersivity of laminated Cr2AlC is good for 0.6 wt% concentration, together with the micro & nano bearing effect, so as to form a layer of tribofilm, and result in a decrease of the friction coefficient. | ||
| Fig. 8 (a) Friction coefficient as a function of sliding distance, (b) wear scar width on disc specimens lubricated with different concentrations Cr2AlC in 100SN base oil. | ||
Fig. 8(b) gives the wear scar width (WSW) vs. the different Cr2AlC concentration. It can be seen that the wear scar width of base oil is slight decreased by adding laminated Cr2AlC, except for the base oil containing 5 wt% concentration Cr2AlC is obviously higher than that of other sample oil, which is in good accordance with the friction coefficient value in Fig. 8(a). Therefore, the optimum concentration of the synthesized Cr2AlC as an additive in base oil is suggested to be 0.6 wt%.
In this work, it has been shown that the base oil with a certain viscosity containing 0.6 wt% Cr2AlC can form a certain thickness tribofilm, which can decrease shearing stress, therefore, give a low friction coefficient and wear scar width. In the friction process, because of the contact pressure creating traction-compression stressed zones, a thin tribofilm is formed on the metal substrate, the tribofilm could not only withstand the load of the steel ball but also prevent two mating metal surfaces direct contact.
Fig. 9(a) show the variation of friction coefficients with sliding distances for 0.6 wt% concentration Cr2AlC under different loads, respectively. It can be seen that the friction coefficients of base oil with 0.6 wt% Cr2AlC is stable at about 0.092 under the load of 10 N, increasing load to 20 N, after an obvious slightly running-in stage, the friction coefficients almost remain constant at about 0.103, the friction coefficient continuously increases along with the sliding distance under the load of 30 N.
 | ||
| Fig. 9 (a) Friction coefficient, (b) wear scar width of base oil mixed with 0.6% Cr2AlC additive under different loads at 5 m min−1 for 200 m. | ||
Fig. 9(b) shows the wear scar width (WSW) of 100SN base oil containing 0.6 wt% Cr2AlC at different loads under a speed of 5 m min−1 for 200 m. It can be observed that the WSWs increase gradually with the increase of the applied load. The lubrication of Cr2AlC as oil additive is mainly dependent on the formation of tribofilm in the friction process. However, a continuous tribofilm only begins to be formed under an optimal load. With further increase of the load, the friction coefficient has increased due to the extrusion of the tribofilm in the contact zone, and result in a high wear scar width.
Fig. 10(a) displays SEM of the tribofilms formed on the friction surface lubricated by the base oil containing 0.6 wt% synthesized laminated Cr2AlC. The tribofilms were uniform and tenacious on the friction surface, which results in a lower friction and lower wear scar width. In order to confirm the formation of the tribofilm and its composition, the corresponding EDS analysis of the worn surface was carried out. As shown in Fig. 10(b), high intensity peaks from chromium, aluminum, and carbon atoms indicated the formation of an adherent Cr2AlC tribofilm. It is believed that the smooth and flat surface lubricated by composites results from the deposition of tribofilm on the friction surface.
 | ||
| Fig. 10 (a) SEM images of the tribofilms formed on the surface (15 N, 250 rpm, 200 m), and (b) EDS spectrum at the surface of point in (a). | ||
4. Conclusion
By the liquid magnetic stirring mixing raw powders, high purity Cr2AlC powder could be pressureless sintering synthesized from Cr, Al and graphite powder at temperature ranged from 1300 to 1400 °C in flowing argon atmosphere. The increase of the Al content in raw materials is helpful to the improvement of Cr2AlC phase content, Al element here is considered as a promoting factor because it provides a liquid circumstance to speed up the solid reaction among Cr, Al and graphite. The introduction of 0.6 wt% laminated Cr2AlC as lubrication additives improve the tribological properties of the base oil, especially in terms of friction reduction and wear resistance. The excellent tribological properties indicate that the as-prepared Cr2AlC will be useful for its further industrial application as oil additive in the future.Acknowledgements
This work was supported by the National Natural Science Foundation of China (51275213, 51302112), Jiangsu National Nature Science Foundation (BK2011534), Jiangsu National Nature Science Foundation for Colleges and Universities (14KJB460012), and Scientific and Technological Innovation Plan of Jiangsu Province (CXLX12_0636).Notes and references
- C. Lange, M. Hopfeld and M. W. Barsoum, Phys. Status Solidi A, 2012, 209, 545 CrossRef CAS PubMed.
- M. W. Barsoum, Prog. Solid State Chem., 2000, 28, 201 CrossRef CAS.
- Z. M. Sun, Int. Mater. Rev., 2011, 56, 143 CrossRef CAS PubMed.
- S. B. Li, H. L. Li and Y. Zhou, J. Eur. Ceram. Soc., 2014, 34, 1083 CrossRef CAS PubMed.
- M. Xue, H. Tang and C. S. Li, Adv. Appl. Ceram., 2014, 113, 245 CrossRef CAS PubMed.
- D. Li, Y. Liang and X. X. Liu, J. Eur. Ceram. Soc., 2010, 30, 3227 CrossRef CAS PubMed.
- X. H. Wang and Y. C. Zhou, J. Mater. Chem., 2002, 12, 2781 RSC.
- W. B. Tian, P. L. Wang and G. Zhang, Mater. Sci. Eng., A, 2007, 454, 132 CrossRef PubMed.
- L. O. Xiao, S. B. Li and G. M. Song, J. Eur. Ceram. Soc., 2011, 31, 1497 CrossRef CAS PubMed.
- J. D. Hettinger, S. E. Lofland and P. Finkel, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 115 CrossRef.
- T. H. Scabarozi, S. Amini and O. Leaffer, J. Appl. Phys., 2009, 105, 013543 CrossRef PubMed.
- J. Wang and Y. Zhou, Annu. Rev. Mater. Res., 2009, 39, 415 CrossRef CAS PubMed.
- W. B. Tian, P. L. Wang and Y. M. Kan, J. Mater. Sci., 2008, 43, 2785 CrossRef CAS PubMed.
- Z. J. Lin, M. S. Li and J. Y. Wang, Acta Mater., 2007, 55, 6182 CrossRef CAS PubMed.
- Y. L. Du, Z. M. Sun and H. Hashimoto, J. Appl. Phys., 2011, 109, 063707 CrossRef PubMed.
- W. B. Tian, P. L. Wang and G. Zhang, Scr. Mater., 2006, 54, 841 CrossRef CAS PubMed.
- W. B. Tian, P. L. Wang and G. Zhang, J. Am. Ceram. Soc., 2007, 90, 1663 CrossRef CAS PubMed.
- C. Hu, L. He and J. Zhang, J. Eur. Ceram. Soc., 2008, 28, 1679 CrossRef CAS PubMed.
- S. B. Li, W. B. Yu and H. X. Zhai, J. Eur. Ceram. Soc., 2011, 31, 217 CrossRef CAS PubMed.
- B. Manoun, R. Rgulve and S. K. Saxena, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, l CrossRef.
- D. B. Lee and T. D. Nguyen, J. Alloys Compd., 2008, 464, 434 CrossRef CAS PubMed.
- W. B. Tian, K. Vanmeensel and P. L. Wang, Mater. Lett., 2007, 61, 4442 CrossRef CAS PubMed.
- W. B. Zhou, B. C. Mei and J. Q. Zhu, J. Mater. Sci., 2005, 40, 3559 CrossRef CAS PubMed.
- S. Amini, A. G. Zhou and S. Gupta, J. Mater. Res., 2008, 23, 2157 CrossRef CAS.
- W. B. Tian, Z. M. Sun and Y. L. Du, Mater. Lett., 2008, 62, 3852 CrossRef CAS PubMed.
- Q. Wu, C. S. Li and H. Tang, Appl. Surf. Sci., 2010, 256, 6986 CrossRef CAS PubMed.
- M. A. Elsaeed, F. A. Deorsola and R. M. Rashad, Int. J. Refract. Met. Hard Mater., 2012, 35, 127 CrossRef CAS PubMed.
- S. Gupta, D. Filimonov and T. Palanisamy, Wear, 2008, 265, 560 CrossRef CAS PubMed.
- D. Filimonov, S. Gupta and T. Palanisamy, Tribol. Lett., 2009, 33, 9 CrossRef CAS.
- S. Gupta, D. Filimonov and V. Zaitsev, Wear, 2008, 264, 270 CrossRef CAS PubMed.
- W. B. Tian, P. L. Wang and Y. M. Kan, Mater. Sci. Eng., A, 2007, 443, 229 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2014 |



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: