Unravelling the surface chemical characteristics and nanostructure of MgO/NiO catalyst using positronium probe: positron annihilation lifetime and age momentum correlation study
S. K. Sharma,
K. Sudarshan and
P. K. Pujari*
Radiochemistry Division, Bhabha Atomic Research Centre, Mumbai-400085, India. E-mail: pujari@barc.gov.in
First published on 13th March 2014
Abstract
MgO/NiO catalyst samples with varying NiO loading (5, 10, 20 and 40 wt%) were prepared by mechanical mixing followed by annealing at 800 °C for 1.5 hours. The grain size and crystallinity of the catalyst samples were determined using X-ray powder diffraction. The triplet (ortho) state of positronium (o-Ps) has been used to characterize the nanostructure (nano-dimensional open spaces) and surface chemical characteristics of the grains. The availability of these vacant spaces as well as the surface characteristics of grains are crucial for the catalytic activity of a catalyst. Positron annihilation lifetime spectroscopy measurements showed two o-Ps lifetime components (τ3 and τ4) in both pure MgO as well as NiO powders indicating the presence of two types of open spaces. In the case of the catalyst samples, o-Ps component, τ3, is observed to disappear most probably due to spin-conversion of o-Ps in the presence of paramagnetic Ni2+ at MgO grain surfaces in the form of a NiO/MgO solid solution layer. This phenomenon has been confirmed by measuring positron age dependent line shape S(t)-parameter using the positron age momentum correlation (AMOC) technique. The study indicates the presence of a NiO/MgO solid solution layer on the surface of MgO grains along with other nanostructural changes as a result of mechanical mixing followed by the annealing process.
Introduction
The oxide solid solutions having transition metal oxide as solute and insulating diamagnetic oxide as solvent are used as catalysts for N2O decomposition, CO and CO2 hydrogenation, and steam reforming. MgO/NiO is one of the oxide solution catalysts which have been shown to possess excellent catalytic activity as well as selectivity for CO2 reformation and rather high stability.1–3 This catalyst can inhibit the carbon deposition process in an effective manner compared to other Ni based catalysts.4,5 It has been reported in the literature that catalytic activity and selectivity of this catalyst depends on method of its preparation, Ni loading as well as calcinations temperature.6 Hu and Ruckenstein have studied the methane activation over MgO/NiO catalyst in detail.2,3,6 In their study, it was shown that the activation temperature is lowered in the case of mechanically mixed catalyst over a solid solution catalyst and this was attributed to the possible formation of NiO/MgO solid solution layer on MgO grains as well as defects produced in the bulk of particles. In the field of catalysis, the nanostructure (availability of open spaces) and surface characteristics of grains are very crucial for the efficiency and selectivity of a particular catalyst. This is because the open spaces present in the catalyst provide means to access the active sites. In such a case, it becomes very important to characterize these characteristics of a catalyst.The triplet state of the bound state of a positron and an electron i.e. ortho-positronium (o-Ps) is an efficient probe for quantification of the size and concentration of nano-dimensional open space in porous materials. This happens because positron when implanted in porous materials may form quasi bound state with an electron i.e. Ps, in the form of singlet, para-positronium (p-Ps) and o-Ps in ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The intrinsic annihilation lifetimes of p-Ps and o-Ps are 125 ps and 142 ns, respectively. In porous materials, o-Ps trapped in an open space may collide with the electron from surrounding surface and annihilate with an electron other than its bound partner and opposite spin. This process is called pick-off annihilation and depending on the frequency of collision, the o-Ps lifetime may reduce from 142 ns to 1–10 ns. The details regarding application of positron annihilation techniques for characterization of various materials including porous materials can be found in ref. 7. The technique used to measure positron or positronium lifetime in a sample is called positron annihilation lifetime spectroscopy (PALS). The o-Ps pick-off lifetime, τ3, in a porous material is correlated with the radius of pore, R, (assuming spherical) using the Tao–Eldrup equation.8,9
3. The intrinsic annihilation lifetimes of p-Ps and o-Ps are 125 ps and 142 ns, respectively. In porous materials, o-Ps trapped in an open space may collide with the electron from surrounding surface and annihilate with an electron other than its bound partner and opposite spin. This process is called pick-off annihilation and depending on the frequency of collision, the o-Ps lifetime may reduce from 142 ns to 1–10 ns. The details regarding application of positron annihilation techniques for characterization of various materials including porous materials can be found in ref. 7. The technique used to measure positron or positronium lifetime in a sample is called positron annihilation lifetime spectroscopy (PALS). The o-Ps pick-off lifetime, τ3, in a porous material is correlated with the radius of pore, R, (assuming spherical) using the Tao–Eldrup equation.8,9
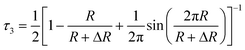 | (1) |
| (τ3,q)−1 = (τ3)−1 + Kq[M] | (2) |
AMOC involves the correlated measurements of positron lifetime and Doppler broadening (DB) of annihilation radiation which enables one to follow momentum distribution of annihilating electron–positron pair as a function of positron age. The information obtained from AMOC helps in the investigation of o-Ps annihilation mode such as spin-conversion, complex formation or simply pick-off annihilation etc. Hence, PALS and DB measurements in combination with AMOC is successful for investigation of nanostructure and surface characteristics of porous materials specifically catalysts wherein surface chemical characteristics have significant importance. It is expected that once the kinetics of o-Ps in a sample is established, the results can be used to quantify the pore size, density and size distribution even in the presence of spin-conversion or complex formation mechanism.
As mentioned before MgO/NiO catalyst is known for its excellent selectivity and activity for CO2 reformation. High surface area is a requirement for the improved efficiency of the catalyst and thus MgO/NiO catalyst prepared using respective powders having nanosize grains is expected to have improved performance. In the present work, we have prepared MgO/NiO catalyst by annealing the mechanically mixed powders. The nanostructure and surface characteristics of the grains have been investigated combining PALS, DB and AMOC measurements. In order to determine the grain size and changes in crystallinity, X-ray diffraction (XRD) measurements have been carried out.
Experimental
The samples were prepared from commercially available pure (>99.9%) MgO and NiO powders. Different mixtures of these two powders with varying content of NiO (5, 10, 20 and 40 wt%) were hand milled using mortar–pestle for more than 2 hours. Thus prepared samples are further referred to as MgO/NiO5, MgO/NiO10, MgO/NiO20 and MgO/NiO40 in the discussion. The catalyst samples were annealed in air atmosphere at 800 °C for 1.5 hours and then furnace cooled to room temperature. Before any further characterization, the annealed mixtures were again hand milled for 30 minutes.The positron lifetime and age momentum correlation measurements were simultaneously carried out at room temperature in laboratory atmosphere using two BaF2 detectors and one HPGe detector.15 The block diagram of the setup is shown in Fig. 1. A 22Na source of high activity (∼1.2 MBq) sandwiched between two Kapton® (7 μm) films was used for the measurements. In order to ensure that all positrons annihilate within the sample, the source was embedded in sufficient amount of powder sample. Two BaF2 detectors placed at 90° angle were used in fast–fast coincidence to record the lifetime spectrum using Time to Amplitude Converter (TAC). The Doppler broadening measurements are carried out using an HPGe detector placed opposite to the BaF2 detector recording stop signal for the lifetime spectrum. The timing signals from all the three detectors are used to produce a master gate using a universal coincidence unit. The coincidence events from TAC and spectroscopy amplifier are recorded using a Computer Aided Measurement and Control (CAMAC) based multi-parameter system. The time resolution of the lifetime spectrometer (part of AMOC setup) and energy resolution of the HPGe detector was measured to be 350 ps (measured using 60Co) and 2.1 keV (1332 keV, 60Co), respectively. As the focus of this study was on the application of o-Ps probe, the time resolution of the setup was kept purposefully on higher side to reduce the counting time for AMOC spectra. The lifetime spectra have been recorded with time dispersion 12.5 ps per channel and 50 ps per channel. Positron lifetime spectrum of reference material (Si crystal) was recorded to evaluate the fraction of positrons annihilating in the Kapton® films and the source itself. All the lifetime spectra have been analyzed using PATFIT-88 as a sum of decaying exponential components.17 In order to analyze the AMOC data, positron age dependent Doppler broadening spectra were obtained by summing the correlated events within a variable window (t1 to t2) on positron age axis. These spectra were analyzed in terms of age dependent line shape parameter S(t), t = (t1 + t2)/2. The S(t) parameter of all samples was calculated by taking ratio of central area (511 ± 1.8 keV) to the total area of the photo peak in order to keep the value ∼0.50 for pure MgO powders sample. There was only one positron component (390 ps) from annihilation of positrons in the source and has been ignored for the evaluation of S(t).
X-ray diffraction measurements were carried out with Philips X pert pro XRD unit using Cu Kα radiation. The scanning was carried out with a step of 0.02° at a rate of 1° min−1.
Results and discussion
The XRD pattern for pure MgO, NiO powders and catalysts samples are shown in Fig. 2. The diffraction patterns for the MgO and NiO powders do not show any impurities and can be identified with pure MgO and NiO lattice. The XRD patterns show that all the samples have grain size in nano-dimension. In case of catalyst samples, no new peak corresponding to any newly formed compounds is seen as a result of annealing. The arrows shown in Fig. 2 in case of MgO/NiO20 and MgO/NiO40 indicate the diffraction from NiO grains. The average grain size D has been calculated using Scherrer's formula18
D = Kλ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ
| (3) |
Fig. 3 shows a typical 3D contour plot of positron lifetime and Doppler broadening spectra of MgO/NiO10 sample obtained from AMOC measurements. The X and Y-projections from the plot taken over full range on X and Y-axis provide positron lifetime spectrum and Doppler broadening spectrum, respectively. Fig. 4 shows such typical positron lifetime spectra for pure MgO powder and MgO/NiO5 sample (X-projection taken from the corresponding 3D plots) indicating a clear change in the o-Ps lifetime on loading of NiO. In case of both pure MgO and NiO powders, three lifetime components have been observed as shown in Table 1. Two longer components (τ3 and τ4) are in nanosecond range and hence attributed to formation and annihilation of o-Ps in the samples. In the present study, the smallest component generally attributed to p-Ps annihilation (τ1 = 125 ps with I1 = Io-Ps/3) has not been resolved due to poor time resolution of the spectrometer and hence, the observed shortest component (τ2) is ascribed to p-Ps annihilation as well as free positron annihilation in vacancy defects or clusters present in the sample. The longer component, τ3, (0.924 and 1.220 ns) in pure MgO and NiO powders, respectively is ascribed to o-Ps pick-off annihilation in open spaces present at the grains boundaries of these powders. The longest component, τ4, in the range of >6 ns observed in both the cases is attributed to o-Ps pick-off annihilation in some large voids present as unoccupied spaces between nanosized grains.19 To examine the presence of other longer components or effect of time range used for the measurements on longest component (τ4), lifetime spectra for all the samples have also been recorded with time dispersion of 50 ps per channel. No significant changes were observed in the longest component (τ4) value and corresponding intensity (I4). The intensity (I4) being very low (of the order of 1%) can be an artifact of PALS analysis or can result from structural inhomogeneity in the samples. Hence, no further discussion is made on the changes in τ4 and I4. The PALS measurements have been carried out in laboratory atmosphere in the presence of air which reduces the o-Ps lifetime in the samples due to oxygen quenching effects. In such a case, combination of eqn (1) and (2) are used to estimate the pore size. For this calculation, Kq and [M] must be accurately known. In the present case, the pore size has been calculated taking Kq = 0.0040 ns−1 atm.−1 and [M] = 0.2 atm. for oxygen present in the air.14 The size (radius) of open spaces (considering spherical) corresponding to τ3 and τ4 available in MgO are 1.53 and 5.94 Å and in NiO are 2.00 and 5.73 Å, respectively. It is to be noted that in case of NiO, spin-conversion due to presence of Ni2+ has not been considered for calculation of size of open spaces. This indicates that the actual size of open spaces in NiO powder can be larger than 2.00 and 5.73 Å and can be determined only after determination of Kq for Ni2+. Similar to pure powders, in all the catalyst samples, three lifetime components have been observed as shown in Table 1. The main difference between pure powders and catalyst samples is that the o-Ps component (τ3) is not observed in case of catalyst samples.
 | ||
| Fig. 3 Typical 3D contour plot of positron lifetime spectrum and Doppler broadening spectrum of MgO/NiO10 sample. | ||
| Sample | τ1/ps, I1/% | τ2/ps, I2/% | τ3/ns, I3/% | τ4/ns, I4/% |
|---|---|---|---|---|
| MgO | — | 340.3 ± 2.3, 83.9 ± 0.7 | 0.924 ± 0.2, 14.4 ± 0.6 | 6.94 ± 0.4, 1.6 ± 0.1 |
| NiO | — | 415.3 ± 1.6, 95.8 ± 0.4 | 1.220 ± 0.12, 2.7 ± 0.3 | 6.8 ± 0.32, 2 ± 0.3 |
| MgO/NiO5 | 282.8 ± 7.2, 71.3 ± 0.4 | 526.5 ± 2.2, 26.8 ± 0.5 | — | 12.9 ± 1.0, 1.7 ± 0.5 |
| MgO/NiO10 | 269.9 ± 8.6, 66.7 ± 0.5 | 501.7 ± 2.0, 31.5 ± 2.7 | — | 10.6 ± 0.7, 1.7 ± 0.4 |
| MgO/NiO20 | 245.9 ± 2.4, 66.0 ± 0.6 | 467.0 ± 3.0, 31.0 ± 2.0 | — | 15.6 ± 0.8, 1.4 ± 0.2 |
| MgO/NiO40 | 266.2 ± 4.8, 68.1 ± 2.7 | 491.5 ± 3.0, 30.6 ± 2.7 | — | 15.0 ± 0.8, 1.2 ± 0.8 |
The Doppler broadening spectra obtained as Y-projection of the AMOC spectra (Fig. 3) have been analyzed to evaluate the line shape (S) parameter by taking ratio of counts in central region (511 ± 1.8 keV) to the total area of the photo peak. The variation of S-parameter as a function of NiO loading is shown in Fig. 5. The increase in S-parameter value for catalyst sample compared to both pure powders can be a result of either higher positronium fraction or larger contribution from low momentum valence electrons (free positron annihilation) due to creation of large size vacancy clusters. The positron annihilation lifetime measurements (Table 1) showed that the positronium fraction has drastically reduced in the catalyst samples. Though, the disappearance of τ3 can be attributed to disappearance of open spaces at grain boundaries, the complete disappearance of open volumes at grain boundaries seems unrealistic.19 Moreover, the increase in S-parameter with reduction in positronium intensities is unlikely to be solely due to creation of larger vacancy clusters (higher values of τ2 as observed from PALS) within the grains. The consistent interpretation of PALS and DB results together is only possible by invoking chemical reactions of Ps (spin-conversion or complex formation) causing the reduction in o-Ps intensity with an increase in S-parameter. When the spin-conversion/complex formation reduces the o-Ps lifetime to an extent that it is not resolvable from free positron lifetime component, a larger free positron lifetime like component, which is a weighted average of free positron and reduced o-Ps lifetime (∼500 ps) is observed from PALS measurements. The conversion of o-Ps into p-Ps through spin-conversion would also result in a higher S-parameter value as observed from DB measurements.
Though invoking spin-conversion was necessary for a consistent interpretation of PALS and DB results, the confirmation of such process taking place in these samples can only be made by positron age resolved momentum distribution measurements. This is mainly because the S-parameter evaluated from conventional Doppler broadening measurements has contribution from all positron/positronium states and the changes in S-parameter can be observed due to different reasons e.g. larger vacancy defect concentration, change in Ps formation fraction or chemical reaction of o-Ps. In order to confirm the role of chemical reactions of Ps in the present samples, age dependent line shape parameter, S(t), has been evaluated from AMOC measurements.
The S(t)-parameter as a function of positron age is plotted in Fig. 6. In case of oxide powders, it is assumed that Ps is formed at surface of grains following sufficient thermalization of positrons.20,21 Thus formed positronium atom is emitted in open spaces between these grains having epithermal energies. The emitted o-Ps is expected to annihilate in free state in these open spaces and undergoes pick-off annihilation on interaction with grain walls under vacuum. The variation of S(t)-parameter as a function of positron age for pure MgO powders shown in Fig. 6 is very much similar to the observed trend in literature.22 The S(t)-parameter beyond positron age > 1 ns, considering the positron lifetimes observed in pure MgO powders (Table 1), primarily represents the contribution from o-Ps annihilation and in the positron age < 1 ns, contribution is primarily from p-Ps (lifetime 125 ps) and free positron (lifetime ∼ 300–400 ps) annihilation. The average S(t)-parameter value for pure MgO powder in the positron age range (1.5–3 ns) is 0.607 ± 0.004 which is marginally higher compared to average value of S(t)-parameter in the positron age range (0–0.6 ns) that is 0.595 ± 0.002. This indicates that in MgO powder, o-Ps undergoes spin-conversion. Under vacuum, the additional quenching of o-Ps becomes only possible at low temperature due to creation of paramagnetic centres at grain surfaces as a result of positron irradiation.21 In the present experiment under ambient conditions, the additional quenching occur due to the paramagnetic oxygen molecules in air. It is to note that the value of S(t) parameter corresponding to spin-converted o-Ps i.e. p-Ps contributing to higher age (> 1 ns) is more even compared to statistically formed p-Ps range (∼ 0–200 ps). The reason for this observation is as follows; the energy of p-Ps formed as a result of spin-conversion after survival of epithermal o-Ps for a longer time (∼ 1 ns) is lower compared to statistically formed epithermal p-Ps (survival time 125 ps).22 It is interesting to note that S(t)-parameter throughout the studied positron age is higher for pure NiO powder compared to MgO. This is in accordance with PALS results (Table 1) where higher free positron (τ2 = 415.3 ps) and o-Ps lifetime (τ3 = 1.220 ns) are observed in pure NiO powder compared to MgO (τ2 = 340.3 ps and τ3 = 0.924) ns. The higher S(t)-parameter and longer positron lifetimes can also be a result of loose packing and higher surface area due to smaller grain size (7 nm) of NiO compared to pure MgO.
 | ||
| Fig. 6 S(t) Parameter as a function of positron age for pure MgO and NiO powders as well as for all the catalyst samples. | ||
In the case of catalyst samples, an increase in S(t)-parameter at higher positron age (> 1 ns) relative to shorter positron age (< 1 ns) is more significant than pure MgO. For example, in case of MgO/NiO5 sample, the average values of S(t)-parameter in the positron age ranges (1.5–3 ns) and (0–0.6 ns) are 0.640 ± 0.006 and 0.617 ± 0.003, respectively. The large changes in the S(t) values indicates the enhancement in spin-conversion mechanism of o-Ps. The observed change is ascribed to presence of paramagnetic Ni2+ species present on the surface of MgO grains as a result of annealing. This indicates that on annealing a NiO/MgO solid solution layer is formed on the surface of MgO grains. In such a case, the positronium emitted from grain surface to open spaces undergoes frequent collision with solid solution layer having paramagnetic Ni2+ and subsequently annihilates as singlet positronium due to spin-conversion. No significant changes in the trends of S(t) vs. positron age as a function of NiO loading have been observed. This suggests a complete quenching phenomenon at the studied lowest concentration i.e. at 5 wt% NiO loading. This observation is also supported by PALS results where no o-Ps component (τ3) has been observed (Table 1) in case of 5 wt% NiO catalyst sample due to reduction in τ3 values as a result of quenching.
Thus the observed higher value of S(t)-parameter in the positron age range (> 1 ns) (Fig. 6) along with the PALS and DB results indicate the formation of NiO/MgO solid solution layer on MgO grain surfaces. The mixture of MgO and NiO in absence of NiO/MgO solid solution layer on MgO would have resulted in a weighted average value of S(t) ranging between pure MgO and NiO. In the present case, the observed values of S(t) for catalyst samples are higher even compared to pure NiO and supports the formation of NiO/MgO solid solution layer over MgO grains. The higher value of S(t) parameter (positron age > 1 ns) for catalyst samples indicates that smaller size of open spaces are present at grain boundaries in catalyst samples compared to pure NiO powder but it can't be completely confirmed from present study.
The presence of NiO/MgO solid solution layer on the surface of MgO grains has been considered by Hu and Ruckenstein6 to explain the improved catalytic activity of the catalyst. They have investigated MgO/NiO mechanical mixture for the CH4 activation via temperature programmed reaction mass spectroscopy (TP-RMS). In that study, a lower activation temperature was observed for the catalyst samples over pure NiO which was explained by considering the formation of NiO/MgO solid solution on MgO grains. The present study provides a direct experimental evidence of formation of NiO/MgO solid solution layer using o-Ps probe. The higher value of S(t)-parameter at shorter positron age range (0–0.6 ns) for catalyst samples indicates the creation of defects in the powders grains where free positron is trapped and annihilate leading to narrower momentum distribution. The grain growth phenomenon has been confirmed from XRD measurements. Thus, in the present study using XRD and o-Ps probe, experimental evidence are obtained for (i) grain growth of powder samples (ii) larger defect concentration (vacancy or vacancy cluster) within grains and most importantly (iii) formation of a solid solution layer of NiO/MgO at the MgO grain surfaces.
Conclusion
The present study confirms the usefulness of o-Ps as a probe for the investigation of nanostructure and surface chemical characteristics of NiO/MgO catalyst particularly regarding the changes occurring in nanostructure and at grain surface as a result of preparation of this catalyst through mechanical mixing process. The study shows the presence of two types of open spaces having average size in the range of 1 and 6 Å in the powders. On annealing, experimental evidence of formation of NiO/MgO solid solution layer on the MgO grain surface has been obtained through o-Ps quenching process (spin-conversion) using PALS and AMOC techniques. The study also confirms the creation of defects (vacancy and vacancy clusters) in grains and increase in size of the nanograins.References
- E. Ruckenstein and Y. H. Hu, Appl. Catal., 1995, 133(1), 149 CrossRef CAS.
- Y. H. Hu and E. Ruckenstein, Catal. Lett., 1996, 36, 145 CrossRef CAS.
- Y. H. Hu, Catal. Today, 2009, 148, 206 CrossRef CAS.
- A. T. Ashcroft, A. K. Cheetham, M. L. H. Green and D. F. P. Vernon, Nature, 1991, 352, 225 CrossRef CAS.
- J. R. Rostrup-Nielsen, Stud. Surf. Sci. Catal., 1998, 36, 73 CrossRef.
- Y. H. Hu and E. Ruckenstein, Langmuir, 1997, 13, 2055 CrossRef CAS.
- Principles and Applications of Positron and Positronium Chemistry, ed. Y. C. Jean, P. E. Mallon and D. M. Schrader, World Scientific, 2003 Search PubMed.
- S. J. Tao, J. Chem. Phys., 1972, 56, 5499 CrossRef CAS.
- M. Eldrup, D. Lightbody and J. N. Sherwood, Chem. Phys., 1981, 63, 51 CrossRef CAS.
- G. Duplatre and I. Billard, in Principles and Applications of Positron and Positronium Chemistry, ed. Y. C. Jean, P. E. Mallon and D. M. Schrader, World Scientific, 2003, p. 73 Search PubMed.
- T. Hyodo, T. Nakayama, H. Saito, F. Saito and K. Wada, Phys. Status Solidi C, 2009, 6, 2497 CrossRef CAS.
- R. A. Ferrell, Phys. Rev., 1957, 108, 167 CrossRef CAS.
- G. Duplatre, A. Hesseler and J. Ch. Abbe, J. Phys. Chem., 1985, 89, 1756 CrossRef CAS.
- K. Sudarshan, D. Dutta, S. K. Sharma, A. Goswami and P. K. Pujari, J. Phys.: Condens. Matter, 2007, 19, 386204 CrossRef.
- S. K. Sharma, J. Prakash, K. Sudarshan, P. Maheshwari, D. Sathiyamoorthy and P. K. Pujari, Phys. Chem. Chem. Phys., 2012, 14, 10972 RSC.
- H. Stoll, P. Castellaz and A. Siegle, in Principles and Applications of Positron and Positronium Chemistry, ed. Y. C. Jean, P. E. Mallon and D. M. Schrader, World Scientific, 2003, p. 349 Search PubMed.
- P. Kirkegaard and M. Eldrup, Comput. Phys. Commun., 1991, 17, 401 Search PubMed.
- B. D. Cullity, Elements of X-ray diffraction, Addison-Wesley, Philippines, 1978, p. 284 Search PubMed.
- H. J. Zhang, Z. Q. Chen and S. J. Wang, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 82, 035439 CrossRef.
- W. Brandt and R. Paulin, Phys. Rev. Lett., 1968, 21, 193 CrossRef CAS.
- C. Dauwe, B. van waeyenberge, D. Segers, T. Van Hoecke and J. Kuriplach, J. Radioanal. Nucl. Chem., 1996, 210, 293 CrossRef CAS.
- B. van Waeyenberge, C. Dauwe and H. Stoll, Mater. Sci. Forum, 2001, 363, 401 CrossRef.
| This journal is © The Royal Society of Chemistry 2014 |




