Novel choline-chloride-based deep-eutectic-solvents with renewable hydrogen bond donors: levulinic acid and sugar-based polyols
Zaira
Maugeri
and
Pablo
Domínguez de María
*
Institut für Technische und Makromolekulare Chemie (ITMC), RWTH Aachen University, Worringerweg 1, 52074, Aachen, Germany. E-mail: dominguez@itmc.rwth-aachen.de; Fax: +49 241 8022177; Tel: +49 241 8020468
First published on 18th November 2011
Abstract
Choline chloride (ChCl) has been combined with renewable hydrogen bond donors (HBD) to form novel deep eutectic solvents (DES) with melting points lower than 100 °C. In some cases (e.g. sorbitol or isosorbide as HBDs) chiral DES can be easily produced. Moreover, the addition of glycerol decreases the viscosity of DES significantly. In the ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) levulinic acid DES case, singular properties were observed. Thus, equimolecular mixtures of ChCl
levulinic acid DES case, singular properties were observed. Thus, equimolecular mixtures of ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) levulinic acid (1
levulinic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) formed a crystal at room temperature, rapidly melting in contact with air humidity. Addition of excess of acetone to ChCl
1) formed a crystal at room temperature, rapidly melting in contact with air humidity. Addition of excess of acetone to ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) levulinic acid (1
levulinic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) leads to the almost quantitative precipitation of choline chloride, which can be used again to form a DES.
2) leads to the almost quantitative precipitation of choline chloride, which can be used again to form a DES.
Deep eutectic solvents (DES) form by complexion of quaternary ammonium salts (e.g. choline chloride, ChCl) with hydrogen bond donors (HBDs) such as amines, amides, alcohols, or carboxylic acids.1–5 Actually, the interaction of the HBD with the quaternary salt reduces the anion–cation electrostatic force, thus decreasing the freezing point of the mixture. DES share many features of conventional ionic liquids, yet DES components are often cheaper and biodegradable. Promising applications of DES have already been reported, e.g. in electrochemistry and electrodeposition,6–9 as (co-)solvents in organic10 and inorganic syntheses,11 as well as in biocatalysis.3,4,12–13
Herein the formation of novel choline-chloride-based deep-eutectic-solvents is reported. To this end, several bio-based HBDs such as carboxylic acids and saccharide-derived polyols were assessed. Interestingly, some of them (e.g.D-sorbitol, isosorbide) may form chiral DES in a straightforward manner. Renewable and cheap chiral deep-eutectic-solvents might represent a useful entry to, e.g. carry out enantioselective reactions.
DES were formed by gently stirring the salt and the HBD at 100 °C until a clear, homogenous liquid formed (typically between 0.5–2 h). Different ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD ratios were tested (from 1
HBD ratios were tested (from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 to 1
0.5 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mol
2 mol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mol) to assess which was the proper combination that would lead to the eutectic depression. Remarkably, all DES formed showed melting points below 100 °C. In Table 1 the best choline chloride
mol) to assess which was the proper combination that would lead to the eutectic depression. Remarkably, all DES formed showed melting points below 100 °C. In Table 1 the best choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD combinations are summarized. Depending on the HBD, different proportions (compared to ChCl) were needed to achieve the melting point depression.
HBD combinations are summarized. Depending on the HBD, different proportions (compared to ChCl) were needed to achieve the melting point depression.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD molar ratios. The molar ratio in each case represents the best combination found to reach the deepest melting point depression. (Mp*): melting point of pure HBD. (Mp): melting point of produced DES. RT = room temperature
HBD molar ratios. The molar ratio in each case represents the best combination found to reach the deepest melting point depression. (Mp*): melting point of pure HBD. (Mp): melting point of produced DES. RT = room temperature
| Hydrogen bond donor (HBD) | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD ratio (mol HBD ratio (mol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mol) mol) |
Mp*/°C | Mp/°C | |
|---|---|---|---|---|
|
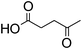
Levulinic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
32 | Liquid at RT |
|
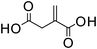
Itaconic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
166 | 57 ± 3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
||||
|
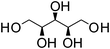
Xylitol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
96 | Liquid at RT |
|
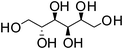
D-Sorbitol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 | Liquid at RT |

|
L-(+)-Tartaric acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
171 | 47 ± 3 |

|
D-Isosorbide | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
62 | Liquid at RT |

|
4-Hydroxybenzoic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
215 | 87 ± 3 |

|
Caffeic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
212 | 67 ± 3 |
|
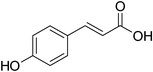
p-Coumaric acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
214 | 67 ± 3 |

|
trans-Cinnamic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
133 | 93 ± 3 |

|
Suberic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
142 | 93 ± 3 |

|
Gallic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
251 | 77 ± 3 |
Recently it was reported that mixing choline chloride with glycerol leads to a diminished viscosity and density of the mixture, providing evidence that beyond a simple mixture of chemicals, actually a DES is formed.14 When addition of glycerol to the above reported DES was assessed, it actually turned out that less viscous derivatives were achieved (Table 2). In the case of suberic acid and gallic acid (entries 7, 8), experiments with glycerol were performed with mixtures in which no DES could be formed (both components, choline chloride and HBD remained solid at 100 °C). As observed, the addition of glycerol led to the formation of solutions with lower melting points. Remarkably, the use of ternary mixtures to form DES (ChCl, HBD and glycerol as second HBD) may significantly enhance the possibilities that DES may bring, in terms of combinations and tuned properties. Until now research in this line has attracted very little attention.14,15
| Entry | HBD | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD HBD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gly ratio Gly ratio |
Mp#/°C without glycerol | Mp/°C with glycerol |
|---|---|---|---|---|
| 1 | Itaconic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
57 ± 3 | Liquid at RT |
| 2 | L-(+)-Tartaric acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 0.25 |
47 ± 3 | Liquid at RT |
| 3 | 4-Hydroxybenzoic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 0.25 |
87 ± 3 | 63 ± 3 |
| 4 | Caffeic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
67 ± 3 | Liquid at RT |
| 5 | p-Coumaric acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 0.25 |
67 ± 3 | 63 ± 3 |
| 6 | trans-Cinnamic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
93 ± 3 | 87 ± 3 |
| 7 | Suberic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
No DES formed | 73 ± 3 |
| 8 | Gallic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 0.25 |
No DES formed | 53 ± 3 |
As observed (Table 1), saccharides and derived polyols can be used as HBD to form DES as well. Importantly, sugar-based DES exhibit a neutral pH in the mixture, what may be very useful for envisaging DES applications. Yet, an important drawback of these solvents is their high viscosity, especially at mild temperatures. Therefore, the influence of the glycerol on viscosities was further studied using an MCR 301 rheometer from Anton Paar with a thermostated jacket (viscosity range 130–34400 cP) (Table 3).
| DES | Viscosity/cP | |||
|---|---|---|---|---|
| 303.15 K | 323.15 K | 343.15 K | 373.15 K | |
ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glucose (1 glucose (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
N.d. | 34400 | 4470 | 560 |
ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glucose glucose![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol (1 glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5) 0.5) |
4430 | 930 | 280 | N.d. |
ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xylitol (1 xylitol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
5230 | 860 | 250 | N.d. |
ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xylitol xylitol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol (1 glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5) 0.5) |
1420 | 370 | 130 | N.d. |
ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sorbitol (1 sorbitol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
12730 | 2000 | 480 | N.d. |
ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sorbitol sorbitol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol (1 glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5) 0.5) |
1710 | 560 | 180 | N.d. |
As observed (Table 3), the viscosity changed significantly as a function of the temperature, the sugar, and the addition of glycerol to the mixture. To study the effect further, the variation of viscosity, η, with the temperature was followed by eqn (1).
| ln η = ln η0 + Eη/RT | (1) |
 | ||
| Fig. 1 Variation of viscosity depending on the temperature of choline-chloride-based DES with different HBDs, upon glycerol addition (25 mol%). | ||
All formed DES had initial water contents of 0.3–1.7 wt% (Karl Fischer). Interestingly, the choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) levulinic acid (ChCl
levulinic acid (ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA) mixture led to singular properties. Thus, ChCl
LA) mixture led to singular properties. Thus, ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA (1
LA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) resulted in a liquid DES at room temperature (Table 1). However, at equimolar proportions (1
2) resulted in a liquid DES at room temperature (Table 1). However, at equimolar proportions (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) the mixture formed a white crystal at room temperature. In contact with air humidity the mixture became rapidly liquid. Qualitatively, when a thin layer (∼1 mm thickness) was spread over a glass Petri dish, the solid became completely liquid within one hour (Fig. 2). Water content was ∼1.2 wt% in the solid DES, increasing up to ∼9.5 wt% in the liquid form. Thus, such a DES might find applications as a biodegradable and inexpensive material for, e.g. humidity sensors. Once the water was removed (by heating the liquid mixture at 100 °C in an open vessel), and the DES was cooled down back to room temperature, the crystal was formed again.
1) the mixture formed a white crystal at room temperature. In contact with air humidity the mixture became rapidly liquid. Qualitatively, when a thin layer (∼1 mm thickness) was spread over a glass Petri dish, the solid became completely liquid within one hour (Fig. 2). Water content was ∼1.2 wt% in the solid DES, increasing up to ∼9.5 wt% in the liquid form. Thus, such a DES might find applications as a biodegradable and inexpensive material for, e.g. humidity sensors. Once the water was removed (by heating the liquid mixture at 100 °C in an open vessel), and the DES was cooled down back to room temperature, the crystal was formed again.
 | ||
Fig. 2 Layers of ∼1 mm thickness of ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA (1 LA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). In both glass Petri dishes the same amount of DES was added, and then one was closed. After 2 min, the first liquid drops appeared in the open sample. Within 60 min the solid was already completely liquid. 1). In both glass Petri dishes the same amount of DES was added, and then one was closed. After 2 min, the first liquid drops appeared in the open sample. Within 60 min the solid was already completely liquid. | ||
To study the effect quantitatively, gravimetric determinations of water absorption kinetics were carried out for two different ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA DES (1
LA DES (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) in a closed system at a specific humidity level (50 ± 3%).
2) in a closed system at a specific humidity level (50 ± 3%).
The initial water content in the DES was measured by Karl Fischer titration (∼1.1–1.2 wt%). This value was converted into a molar ratio of water and used as the starting point (0 min). A precise amount of DES was put in a closed glass Petri dish (Fig. 3) and the weight variation was measured over time with a balance (0.1 mg precision) (Fig. 4).
 | ||
| Fig. 3 Closed system for the gravimetric determination of the water absorption. The surface exposed to the air was 804 mm2 (diameter of the glass Petri dish 32 mm). | ||
 | ||
Fig. 4 Water absorption kinetics of ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA. (●) 1 LA. (●) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; (■) 1 2; (■) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
The obtained data (Fig. 4) fitted well with eqn (2) (R2 > 0.97), recently proposed for measuring the water absorption by ionic liquids.16
| M = M∞ (1 − e−kt) | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA (1
LA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) turned out to be less hydrophilic than ChCl
1) turned out to be less hydrophilic than ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA (1
LA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (Table 4).
2) (Table 4).
| DES | k/h−1 | M ∞ | kM ∞/h−1 | R 2 |
|---|---|---|---|---|
ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA (1 LA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.0314 | 219.0 | 6.88 | 0.9860 |
ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA (1 LA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
0.0512 | 276.1 | 14.14 | 0.9770 |
All herein reported liquid DES (Table 1) formed two phases with aprotic bio-based organic solvents such as acetone, ethyl acetate, or 2-methyltetrahydrofuran, whereas DES were soluble in water and in protic solvents such as methanol. Surprisingly the ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA (1
LA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) DES led again to singular results. By adding acetone to that DES, choline chloride immediately precipitated, being almost quantitatively recovered upon filtration. Thus, the addition of acetone disrupts the interaction between choline chloride and levulinic acid, breaking the DES interaction, and therefore precipitating choline chloride. An excess of acetone was needed (>10 equiv.) to afford an almost quantitative recovery of choline chloride (Fig. 5). The purity of the recovered choline chloride was not affected by the precipitation reaction (1H NMR) and was used to produce the DES again by adding levulinic acid and gently stirring at 100 °C. A similar effect was observed when isosorbide was used as HBD.
2) DES led again to singular results. By adding acetone to that DES, choline chloride immediately precipitated, being almost quantitatively recovered upon filtration. Thus, the addition of acetone disrupts the interaction between choline chloride and levulinic acid, breaking the DES interaction, and therefore precipitating choline chloride. An excess of acetone was needed (>10 equiv.) to afford an almost quantitative recovery of choline chloride (Fig. 5). The purity of the recovered choline chloride was not affected by the precipitation reaction (1H NMR) and was used to produce the DES again by adding levulinic acid and gently stirring at 100 °C. A similar effect was observed when isosorbide was used as HBD.
 | ||
Fig. 5 Correlation between equivalents of acetone added to ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA (1 LA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and efficiency in choline chloride recovery. 2) and efficiency in choline chloride recovery. | ||
In summary, a number of novel deep eutectic solvents have been reported. These DES are entirely composed of bio-based and biodegradable components, providing in some cases access to chiral DES. Choline chloride was used as the quaternary salt, whereas several carboxylic acids (aliphatic and aromatic, mono- and dicarboxylic), and saccharide-derived polyols were used as hydrogen bond donors. The further addition of glycerol decreases the viscosities and the melting points of the ternary mixtures. A combinations of ChCl and levulinic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) resulted in a white crystal at room temperature, rapidly melting to a liquid on adsorbing air humidity. A simple equation can be used to describe the water absorption kinetics of levulinic-based DES. Addition of an excess of acetone to the ChCl
1) resulted in a white crystal at room temperature, rapidly melting to a liquid on adsorbing air humidity. A simple equation can be used to describe the water absorption kinetics of levulinic-based DES. Addition of an excess of acetone to the ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA (1
LA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) mixture led to almost quantitative precipitation of choline chloride.
2) mixture led to almost quantitative precipitation of choline chloride.
Acknowledgements
Financial support from DFG training group 1166 “BioNoCo” (“Biocatalysis in Non-conventional Media”) is gratefully acknowledged. We thank Mr Philip Engel and Mr Heiner Giese from Aachener Verfahrenstechnik (RWTH Aachen University) for their assistance in the viscosity measurements.References
- A. P. Abbott, D. Boothby, G. Capper, D. L. Davies and R. K. Rasheed, J. Am. Chem. Soc., 2004, 126, 9142–9147 CrossRef CAS.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2003, 70–71 RSC.
- D. Lindberg, M. de la Fuente Revenga and M. Widersten, J. Biotechnol., 2010, 147, 169–171 CrossRef CAS.
- J. T. Gorke, R. J. Kazlauskas and F. Srienc, US2009/0117628, 2009 Search PubMed.
- A. P. Abbott, G. Capper, D. L. Davies, H. L. Munro, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2001, 2010–2011 RSC.
- M. Figueiredo, C. Gomes, R. Costa, A. Martins, C. M. Pereira and F. Silva, Electrochim. Acta, 2009, 54, 2630–2634 CrossRef CAS.
- R. Costa, M. Figueiredo, C. M. Pereira and F. Silva, Electrochim. Acta, 2010, 55, 8916–8920 CrossRef CAS.
- A. H. Whitehead, M. Pölzler and B. Gollas, J. Electrochem. Soc., 2010, 157, D328–D334 CrossRef CAS.
- K. Haerens, E. Matthijs, A. Chmielarz and B. van der Bruggen, J. Environ. Manage., 2009, 90, 3245–3252 CrossRef CAS.
- A. P. Abbott, T. J. Bell, S. Handa and B. Stoddart, Green Chem., 2005, 7, 705–707 RSC.
- A. P. Abbott, G. Capper, D. L. Davies, K. J. McKenzie and S. U. Obi, J. Chem. Eng. Data, 2006, 51, 1280–1282 CrossRef CAS.
- J. T. Gorke, F. Srienc and R. J. Kazlauskas, Chem. Commun., 2008, 1235–1237 RSC.
- P. Domínguez de María and Z. Maugeri, Curr. Opin. Chem. Biol., 2011, 15, 220–225 CrossRef.
- A. P. Abbott, R. C. Harris, K. S. Ryder, C. D́Agostino, L. F. Gladden and M. D. Mantle, Green Chem., 2011, 13, 82–90 RSC.
- A. P. Abbott, J. Collins, I. Dalrymple, R. C. Harris, R. Mistry, F. Qiu, J. Scheirer and W. R. Wise, Aust. J. Chem., 2009, 62, 341–347 CrossRef CAS.
- F. Di Francesco, N. Calisi, M. Creatini, B. Melai, P. Salvo and C. Chiappe, Green Chem., 2011, 13, 1712–1717 RSC.
| This journal is © The Royal Society of Chemistry 2012 |
