A colorimetric and ratiometric NIR fluorescent turn-on fluoride chemodosimeter based on BODIPY derivatives: high selectivity via specific Si–O cleavage†
Jian
Cao
ab,
Chunchang
Zhao
*a,
Peng
Feng
a,
Yulin
Zhang
a and
Weihong
Zhu
*a
aShanghai Key Laboratory of Functional Materials Chemistry, Key Laboratory for Advanced Materials and Institute of Fine Chemicals, East China University of Science & Technology, Shanghai, 200237, P. R. China. E-mail: zhaocchang@ecust.edu.cn; whzhu@ecust.edu.cn
bShanghai University of Engineering Science, Shanghai, 201620, P. R. China.
First published on 18th November 2011
Abstract
A colorimetric and fluorescent turn-on chemodosimeter for fluoride with high selectivity was developed on the basis of the specific reaction of F− with BODIPY-OSi, displaying a dramatic color change and distinct near-infrared (NIR) fluorescence enhancement at 676 nm.
Fluoride ion (F−) is of particular interest owing to its considerable significance for health and environmental issues. It is primarily used in dental care and the clinical treatment for osteoporosis. However, a high ingestion of fluoride can result in dental and skeletal fluorosis, urolithiasis in humans, and even death.1 Ratiometric development in the detection and sensing of F− has become a topical objective in that the signal ratios at two wavelengths are independent of the sensor concentration, the fluctuation in source-light intensity, and the sensitivity of the instrument, being capable of providing built-in correction for environmental effects.2 Moreover, to achieve high sensitivity, it is critical to develop fluorescent probes with a high off/on ratio.3 To date, hydrogen-containing polar groups, such as amides,4–5 ureas and thioureas,6 pyrrole and indole,7 have been widely employed as hydrogen-bonding donors in the design of F− receptors. Fabbrizzi et al.8 have investigated various colorimetric anion receptors containing NH binding sites, indicating that the deprotonation trend is enhanced by the acidity increase of the hydrogen bond donors and the basicity of the anions. However, realization of ratiometric fluorescent measurements for F− is still a challenge, especially with respect to different mechanisms, NIR wavelength, and fast response.9
Boron dipyrromethene (BODIPY) fluorescent dyes have been recognized as promising signal units due to their high fluorescent quantum yields, effective in imaging living cells.10 A few reports have been published concerning BODIPY as a signaling unit for F−, however, most of them show a turn-off response in emission spectra and modest sensitivity.9a Recently, our group has designed a 6-hydroxyindole-based boron dipyrromethene, BODIPY-OH, displaying a drastic spectral shift (about 90 nm) by controlling the phenol/phenolate interconversion.11 Because of the incorporated larger conjugation of 6-hydroxyindole in the BODIPY unit, the fluorophore in the anion form is highly fluorescent around 676 nm (falling in the NIR region) when excited at 644 nm, whereas the fluorophore in the neutral form is essentially non-fluorescent under the same conditions. The phenomenon can be useful for designing a colorimetric and NIR fluorescent turn-on probe with a high off/on ratio. With this in mind, here we report a fluoride sensor, BODIPY-OSi (Fig. 1a), exploiting the extraordinary affinity of fluoride for silicon. As demonstrated, the release of anion form BODIPY-O−via a fluoride-induced Si–O bond cleavage can cause a large redshift in the absorbance, showing a distinct turn-on NIR fluorescence with a high off/on ratio, preferable to realize minimal interfering absorption and fluorescence from biological samples, low scattering, and deep penetration into tissues. Remarkably, the chemodosimeter shows several favorable characteristics in the beneficial sense, such as fast response, and naked-eye detectable chromogenic and fluorogenic dual responses to F−. To the best of our knowledge, BODIPY-OSi is the first NIR fluorescent turn-on sensor of fluoride with both colorimetric and ratiometric functions.
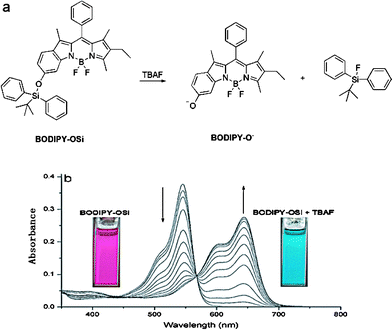 | ||
| Fig. 1 (a) F−-promoted signaling mechanism. (b) Absorption spectra of BODIPY-OSi (5 × 10−6 M) in the presence of different concentrations of TBAF (0, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 30, 50 equiv.) in CH2Cl2 solution. Insets: color changes of BODIPY-OSi upon addition of TBAF (20 equiv.). | ||
BODIPY-OSi was readily synthesized in one step by reaction of BODIPY-OH with tert-butyldiphenylchlorosilane in the presence of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) in a reasonable yield (Scheme S1†), and fully characterized by 1H NMR, 13C NMR and HRMS.
The sensory response of the BODIPY-OSi probe is exemplified by its reaction with F− in CH2Cl2 (Fig. 1b) at room temperature. A fascinating feature is that the reaction of BODIPY-OSi with tetrabutylammonium fluoride (TBAF) triggers a 100 nm redshift in absorbance, attributable to the F−-promoted deprotection of BODIPY-OSi liberating BODIPY-O− (Fig. 1a). As shown in Fig. 1b, upon titration with TBAF to a solution of BODIPY-OSi in CH2Cl2, a decrease in the absorption band at 546 nm and a concomitant increase of a new band at 644 nm were observed, with a distinct isosbestic point at 567 nm, resulting in a color change from pink to indigo. The absorption band at 644 nm is the characteristic absorption of BODIPY-O−,11 which is distinctly red-shifted by 100 nm compared to that of BODIPY-OSi. Notably, the ratio of the absorbance at 644 and 546 nm increases over 3000-fold, allowing the possible colorimetric and ratiometric detection of F− even with the naked eye.
Next we assessed the ability of BODIPY-OSi to detect F− by fluorescence measurement. Upon the addition of TBAF, BODIPY-OSi exhibits different emission behaviors when excited with different wavelengths. Free BODIPY-OSi shows strong fluorescence at about 573 nm, and no visible variations are observed in the assay condition, suggesting that BODIPY-OSi is stable and not converted to BODIPY-O−. Since free BODIPY-OSi shows an absorption band at 546 nm and no characteristic absorption at 644 nm, no emission band at 676 nm was observed with the excitation wavelength at 644 nm. However, the introduction of F− (20 equiv. of F−) elicits a dramatic increase in the emission around 676 nm with a large fluorescence enhancement factor I/I0 (216-fold) due to the new F−-triggered absorption at 644 nm (Fig. 2a). Apparently, the chemodosimeter preferably exhibits a high fluorescent off/on ratio with the large redshift of the absorbance triggered by F− ions. Notably, upon addition of F−, a pronounced enhancement at 676 nm was noted even after 30 s, indicative of a fast reaction with F− (Fig. S1†). Moreover, when excited at the isosbestic point of 567 nm, the emission intensity at 573 nm decreases gradually with the simultaneous appearance of a new red-shifted emission band at 676 nm, resulting in the fluorescence color changes from yellow to pink under illumination with a UV lamp (Fig. 2b, inset). Notably, the ratio of emission intensities at 676 and 573 nm (I676/I573) increases from 0.13 to 9.3, showing a 71-fold ratiometric enhancement (Fig. 2b, inset), indicative of an efficient ratiometric response of BODIPY-OSi to F−. Consequently, by monitoring the dual emission with an isosbestic absorption point as single excitation source, BODIPY-OSi can be successfully constructed as a NIR ratiometric F− chemodosimeter, which allows for fast and accurate measurements with elimination of the influence of dye concentration and microenvironmental fluctuations in pH value, refractive index and photobleaching.
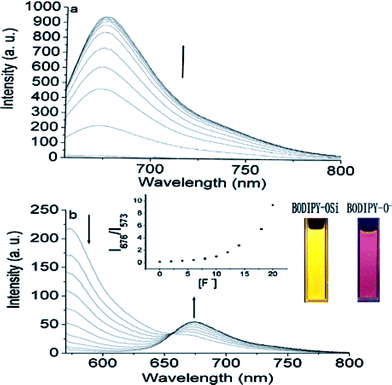 | ||
| Fig. 2 Fluorescence spectra of BODIPY-OSi (5 × 10−6 M) in the presence of different concentrations of TBAF (0, 2, 4, 6, 8, 10, 12, 16, 18, 20 equiv.): (a) excited at 644 nm; (b) excited at the isosbestic point of 567 nm. Inset: ratiometric calibration curve of I676/I573 as a function of TBAF concentration (0–18 equiv.) and fluorescence color changes of BODIPY-OSi. | ||
The UV-vis and fluorescence spectra of the BODIPY-OSi system upon the interaction with 20 equiv. of TBAF are attributable to the resulting BODIPY-O− (Fig. 1a). When F− selectively attacks silicon atoms of the silyl ether group, an increased negative charge on the phenolate oxygen atom induces the release of BODIPY-O−. This mechanism is further confirmed by identifying the same mass of BODIPY-OSi + F− as that of BODIPY-O− in CH2Cl2 (Fig. S2†). In UPLC-mass spectra, BODIPY-OSi gave a retention time at 6.18 min, and a peak due to [M − H]− with 641.2878. Likewise, BODIPY-OSi + F− gave a retention time at 4.02 min, and peak due to [M]− with 403.1793, corresponding to that of BODIPY-O−.
Reactions of F−-promoted Si–O bond cleavage are highly selective for fluoride. As expected, the selectivity of BODIPY-OSi towards F− over relevant anions is impressively high, exemplified though studies of the optical spectra upon addition of excess amounts of various anions. The unique large absorption redshift with the appearance of the characteristic absorption of BODIPY-O− was observed only with the addition of F−. On the other hand, no change in absorption spectra at 644 nm was found with other anions such as Cl−, Br−, I−, HSO4−, HPO42−, H2PO4−, N3−, and NO3− (Fig. 3a). Likewise, BODIPY-OSi turned from pink to indigo upon addition of TBAF but other anions did not induce color changes (Fig. 3c). The detection limit of BODIPY-O− towards F− is evaluated to be 1.18 × 10−7 M, which is comparable or superior to most of the reported F− sensors.9a,12
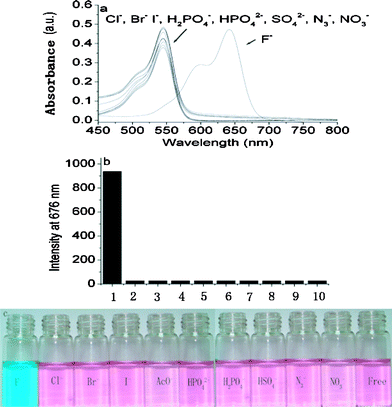 | ||
| Fig. 3 (a) Absorption spectra of BODIPY-OSi (5 × 10−6 M) in the absence and presence of 50 equiv. of various anions. (b) Fluorescence intensity of BODIPY-OSi (5 × 10−6 M) at 676 nm in the absence and presence of 50 equiv. of various anions in CH2Cl2 solution: (1) F−, (2) Cl−, (3) Br−, (4) I−, (5) HSO4−, (6) NO3−, (7) N3−, (8) H2PO4−, (9) HPO42−, (10) free. Each measurement was conducted after 1 min of mixing. (c) Color changes of BODIPY-OSi after the addition of 50 equiv. of various anions. | ||
The fluorescence response of BODIPY-OSi to various anions and its selectivity for F− are shown in Fig. 3b in the form of a bar graph. BODIPY-OSi is non-fluorescent at 676 nm, and the fluorescence change does not take place upon addition of 50 equiv. of Cl−, Br−, I−, HSO4−, HPO42−, H2PO4−, N3−, and NO3−. Only when F− is added, is the unique fluorescent characteristic of BODIPY-O− detected. These results are indicative of the excellent selectivity of BODIPY-OSi towards F− over other competitive anions.
In conclusion, a novel 6-hydroxyindole-based boron dipyrromethene based NIR fluorescent chemodosimeter BODIPY-OSi for F− has been developed with high selectivity via specific Si–O cleavage, which exhibits several advantages: (i) a dramatic color change from pink to indigo, allowing the colorimetric detection of F− by the naked eye; (ii) fast response within 30 s; (iii) the incorporated larger conjugation of 6-hydroxyindole in the BODIPY unit resulting in a NIR fluorescence probe with emission at 676 nm; (iv) turn-on fluorescence to obtain the maximum signal-to-noise ratio, and high ratiometric fluorescent determination with an internal standard using the isosbestic point at 567 nm as excitation wavelength to avoid troublesome calibration. In addition, the developed probe features dual spectroscopic signals and shows extreme selectivity for F− over other anions.
This work was financially supported by NSFC/China, the Oriental Scholarship, SRFDP 200802510011, the Fundamental Research Funds for the Central Universities (WK1013002), the Scientific Research Foundation for the Returned Overseas Chinese Scholars (State Education Ministry) and Foundation from Shanghai University of Engineering Science (A-2500-10-01006).
References
- K. L. Kirk, Biochemistry of the Elemental Halogens and Inorganic Halides, Plenum Press, New York, 1991 Search PubMed.
- (a) K. Kiyose, H. Kojima and T. Nagano, Chem.–Asian J., 2008, 3, 506–515 CrossRef CAS; (b) X. J. Peng, Y. K. Wu, J. L. Fan, M. Z. Tian and K. L. Han, J. Org. Chem., 2005, 70, 10524–10531 CrossRef CAS; (c) Y. Qu, J. Hua and H. Tian, Org. Lett., 2010, 12, 3320–3223 CrossRef CAS.
- (a) J. S. Wu, W. M. Liu, J. C. Ge, H. Y. Zhang and P. F. Wang, Chem. Soc. Rev., 2011, 40, 3483–3495 RSC; (b) W. B. Chen, S. A. Elfeky, Y. Nonne, L. Male, K. Ahmed, C. Amiable, P. Axe, S. J. Yamada, T. D. James, S. D. Bull and J. S. Fossey, Chem. Commun., 2011, 47, 253–255 RSC; (c) S. A. Elfeky, F. D'Hooge, L. Poncel, W. B. Chen, S. P. Perera, J. M. H. van den Elsen, T. D. James, A. T. A. Jenkins, P. Cameron and J. S. Fossey, New J. Chem., 2009, 33, 1466–1469 RSC.
- (a) M. Cametti and K. Rissanen, Chem. Commun., 2009, 2809–2829 RSC; (b) Z. Xu, S. K. Kim and J. Yoon, Chem. Soc. Rev., 2010, 39, 1457–1466 RSC; (c) S. Kumar, V. Luxami and A. Kumar, Org. Lett., 2008, 10, 5549–5552 CrossRef CAS; (d) J. L. Fillaut, J. Andriès, L. Toupet and J. P. Desvergne, Chem. Commun., 2005, 2924–2926 RSC.
- (a) P. Piatek and J. Jurczak, Chem. Commun., 2002, 2450–2451 RSC; (b) S. O. Kang, J. M. Llinares, D. Powell, D. VanderVelde and K. Bowman-James, J. Am. Chem. Soc., 2003, 125, 10152–10153 CrossRef CAS; (c) S. O. Kang, D. Powell and K. Bowman-James, J. Am. Chem. Soc., 2005, 127, 13478–13479 CrossRef CAS; (d) S. O. Kang, Md. A. Hossain, D. Powell and K. Bowman-James, Chem. Commun., 2005, 328–330 RSC; (e) S. O. Kang, D. Powell, V. W. Day and K. Bowman-James, Angew. Chem., Int. Ed., 2006, 45, 1921–1925 CrossRef CAS; (f) S. Xu, K. Chen and H. Tian, J. Mater. Chem., 2005, 15, 2676–2680 RSC; (g) Y. Li, L. Cao and H. Tian, J. Org. Chem., 2006, 71, 8279–8282 CrossRef CAS.
- (a) A. F. Li, J. H. Wang, F. Wang and Y. B. Jiang, Chem. Soc. Rev., 2010, 39, 3729–3745 RSC; (b) F. Han, Y. Bao, Z. Yang, T. M. Fyles, J. Zhao, X. Peng, J. Fan, Y. Wu and S. Sun, Chem.–Eur. J., 2007, 13, 2880–2892 CrossRef CAS; (c) C. Pérez-Casas and A. K. Yatsimirsky, J. Org. Chem., 2008, 73, 2275–2284 CrossRef; (d) Y. Zhao, C. Zhao, L. Wu, L Zhang, C. Tung and Y. Pan, J. Org. Chem., 2006, 71, 2143–2146 CrossRef CAS; (e) S. K. Kim and J. Y. Yoon, Chem. Commun., 2002, 770–771 RSC; (f) M. Vázquez, L. Fabbrizzi, A. Taglietti, R. M. Pedrido, A. M. González-Noya and M. R. Bermejo, Angew. Chem., Int. Ed., 2004, 43, 1962–1965 CrossRef; (g) E. J. Cho, J. W. Moon, S. W. Ko, J. Y. Lee, S. K. Kim, J. Yoon and K. C. Nam, J. Am. Chem. Soc., 2003, 125, 12376–12377 CrossRef CAS.
- (a) P. A. Gale, Chem. Commun., 2008, 4525–4540 RSC; (b) Q. G. Wang, Y. S. Xie, Y. B. Ding, X. Li and W. H. Zhu, Chem. Commun., 2010, 46, 3669–3671 RSC; (c) K.-J. Chang, D. Moon, M. S. Lah and K.-S. Jeong, Angew. Chem., Int. Ed., 2005, 44, 7926–7929 CrossRef CAS; (d) H. Miyaji, W. Sato and J. L. Sessler, Angew. Chem., Int. Ed., 2000, 39, 1777–1780 CrossRef CAS.
- (a) V. Amendola, D. Esteban-Gómez, L. Fabbrizzi and M. Licchelli, Acc. Chem. Res., 2006, 39, 343–353 CrossRef CAS; (b) C. B. Black, B. Andrioletti, A. C. Try, C. Ruiperez and J. L. Sessler, J. Am. Chem. Soc., 1999, 121, 10438–10439 CrossRef CAS.
- (a) O. A. Bozdemir, F. Sozmen, O. Buyukcakir, R. Guliyev, Y. Cakmak and E. U. Akkaya, Org. Lett., 2010, 12, 1400–1403 CrossRef CAS; (b) Y. M. Li, X. L. Zhang, B. C. Zhu, J. L. Yan and W. P. Xu, Anal. Sci., 2010, 26, 1077–1080 CrossRef CAS; (c) K. M. K. Swamy, Y. J. Lee, H. N. Lee, J. H. Chun, Y. Kim, S. J. Kim and J. Yoon, J. Org. Chem., 2006, 71, 8626–8628 CrossRef CAS.
- (a) G. Ulrich, R. Ziessel and A. Harriman, Angew. Chem., Int. Ed., 2008, 47, 1184–1201 CrossRef CAS; (b) A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4891–4932 CrossRef CAS.
- (a) C. Zhao, Y. Zhou, Q. Lin, L. Zhu, P. Feng, Y. Zhang and J. Cao, J. Phys. Chem. B, 2011, 115, 642–647 CrossRef CAS; (b) C. Zhao, P. Feng, J. Cao, Y. Zhang, X. Wang, Y. Yang, Y. Zhang and J. Zhang, Org. Biomol. Chem. 10.1039/c1ob06200j.
- (a) S. Y. Kim and J.-I. Hong, Org. Lett., 2007, 9, 3109–3112 CrossRef CAS; (b) J. F. Zhang, C. S. Lim, S. Bhuniya, B. R. Cho and J. S. Kim, Org. Lett., 2011, 13, 1190–1193 CrossRef CAS; (c) R. Hu, J. Feng, D. Hu, S. Wang, S. Li, Y. Li and G. Yang, Angew. Chem., Int. Ed., 2010, 49, 4915–4918 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: General methods, synthesis, Fig. S1–S2, NMR spectra and HRMS spectrum. See DOI: 10.1039/c1ra00942g |
| This journal is © The Royal Society of Chemistry 2012 |
