Recent advances of pure/independent covalent organic framework membrane materials: preparation, properties and separation applications
Yahui
Cai†
 *a,
Yang
Yu†
a,
Jianfei
Wu
a,
Jiafu
Qu
*a,
Yang
Yu†
a,
Jianfei
Wu
a,
Jiafu
Qu
 b,
Jundie
Hu
b,
Jundie
Hu
 *b,
Dan
Tian
*a and
Jianzhang
Li
*b,
Dan
Tian
*a and
Jianzhang
Li
 a
a
aCo-Innovation Center of Efficient Processing and Utilization of Forest Resources, College of Materials Science and Engineering, Nanjing Forestry University, No. 159 Longpan Road, Nanjing 210037, China. E-mail: yhcai@njfu.edu.cn; tiandan@njfu.edu.cn
bSchool of Materials Science and Engineering, Suzhou University of Science and Technology, Suzhou 215009, China. E-mail: hjd@usts.edu.cn
First published on 4th December 2023
Abstract
Covalent organic frameworks (COF) are porous crystalline polymers connected by covalent bonds. Due to their inherent high specific surface area, tunable pore size, and good stability, they have attracted extensive attention from researchers. In recent years, COF membrane materials developed rapidly, and a large amount of research work has been presented on the preparation methods, properties, and applications of COF membranes. This review focuses on the research on independent/pure continuous COF membranes. First, based on the membrane formation mechanism, COF membrane preparation methods are categorized into two main groups: bottom-up and top-down. Four methods are presented, namely, solvothermal, interfacial polymerization, steam-assisted conversion, and layer by layer. Then, the aperture, hydrophilicity/hydrophobicity and surface charge properties of COF membranes are summarized and outlined. According to the application directions of gas separation, water treatment, organic solvent nanofiltration, pervaporation and energy, the latest research results of COF membranes are presented. Finally, the challenges and future directions of COF membranes are summarized and an outlook provided. It is hoped that this work will inspire and motivate researchers in related fields.
1. Introduction
In 2005, Yaghi1 designed and synthesized porous organic crystal–covalent organic frameworks, highly ordered and periodic network structures connected by covalent bonds of organic building units. These are emerging porous crystalline polymers. COF are mainly composed of light elements such as C, H, O and N. The organic units are connected by covalent bonds. COF have the characteristics of orderly arranged pores, adjustable pore size, high thermal stability, and excellent chemical stability. As a result, COF materials have shown potential for application in many fields and have received a lot of attention from researchers.2–5 However, most COF powder is insoluble, which limits the practical application of COF.6,7The preparation of dispersed COF powders to form continuous COF membrane materials can be a good solution for this problem.8–11 Therefore, in recent years, there are numerous research studies around COF-based composite membrane materials. As shown in Fig. 1, the research related to COF membranes increases from 1 paper in 2012 to 152 papers in 2022. Nevertheless, most of the studies related to COF membranes are on the preparation of COF powders or COF nanosheets with polymers (polyamide, polyimide, polyethersulfone, polyvinylidene fluoride, etc.) to form mixed matrix membranes (MMMs) or thin-film nanocomposites. Although these membranes incorporate COF very well, the mass fraction of COF in the membrane can limit the application performance of the COF, which results in most of the COF-based composite membranes being much inferior to COF powders. This defeats the purpose of preparing COF in membranes for extended applications. In contrast, stand-alone/pure COF membranes have COF as the main body, which allows the role of COF in COF membranes to be maximized.
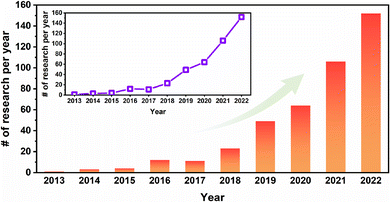 | ||
| Fig. 1 The number of COF membrane-related studies published between 2013 and July 23, 2023. These data were obtained by searching the COF membranes in abstracts on Web of Science. | ||
Therefore, researchers have explored various methods to prepare continuous freestanding/continuous COF membranes, for example, the solvothermal method of continuous COF membranes constructed by directly growing COF in situ on a substrate. Alternatively, researchers explored the interfacial polymerization method to prepare continuous COsF membranes at the interface of two phases.12 Also, some researchers refer to the preparation method of graphene membranes, in which membranes are prepared by the exfoliation of COF powder into layers of COF nanosheets stacked on top of each other.13,14 Traditional amorphous polymer membranes, such as polyamide, polyimide, polyethersulfone, polyvinylidene fluoride, etc., have disadvantages such as the lack of an ordered pore structure, non-uniform pore size as well as fewer pores, limiting their practical applications.15–17 In contrast to conventional polymer membranes, the organic fragments of COF form thermodynamically stable covalent bonds through dynamic and reversible chemical processes. This property acts as a self-healing and error-correcting agent during the synthesis of COF, resulting in a long-range ordered, periodic crystal structure of COF.18,19 Moreover, by changing the organic units, the COF aperture size can be adjusted. For example, the conventional COF aperture size composed of two organic units is uniform, while the COF aperture size composed of three organic units is not uniform, so as to have different selectivity.17,20 In addition, a wider range of applications can be achieved by changing the organic unit to give COF different functional groups and introducing other groups or metal ions after further functionalization.21–26 Independent/pure COF membranes combine the advantages of the COF's ordered arrangement of pore channels, adjustable pore size, high thermal stability, and excellent chemical stability, and are expected to replace polymer membranes in the field of membrane separation technology.
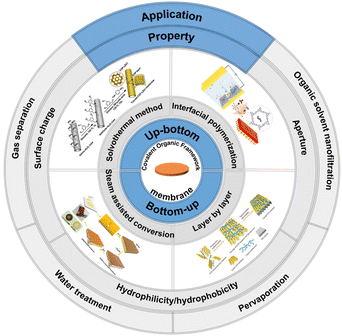
In this review, we aim to introduce and discuss the preparation, properties and applications of COF-based membrane materials, and organize and summarize the research related to COF-based membrane materials in recent years (Fig. 2). Finally, we provide an outlook on the current development status and challenges of COF-based membrane materials.
2. The preparation method of COF membranes
With the in-depth study of COF membranes, the preparation methods of COF membranes have become more diverse, which are no longer limited only to the composite of COF powders in membranes (e.g., COF-based mixed matrix membranes (MMMs), thin-film nanocomposites, etc.), and more researchers are devoted to the study of continuous free-standing/pure COF membranes. This paper summarizes and organizes the preparation methods of COF membranes in recent years. According to the different preparation mechanisms, the preparation methods of COF membranes are categorized into two major groups: bottom-up and top-down. The methods of preparing continuous COF membranes from monomers are collectively called bottom-up, including the solvothermal method and interfacial polymerization method. Methods for preparing COF membranes from COF powder or COF nanosheets are collectively referred to as top-down methods, including steam-assisted conversion and layer-by-layer methods.2.1. Bottom-up
The bottom-up preparation of COF membranes generally involves the in situ growth of COF on a substrate to form a continuous COF membrane. Bottom-up includes the solvothermal method and interfacial polymerization.With the continuous research on COF, the solvothermal method is no longer limited to the preparation of COF powder, but can also be used to prepare continuous COF membranes. To prepare continuous COF membranes, the pretreated substrate can be immersed in the reaction solution, and the COF will accumulate and grow on the surface of the substrate. The continuous COF membranes are obtained by washing with solvent and drying at the end of the reaction (Fig. 3a). The surface of the pretreated substrate has reactive groups such as amino and hydroxyl groups, which allows COF to grow in situ on the substrate. And the strong hydrogen bonding between the COF makes the COF stacked tightly, and then continuous COF membrane materials are prepared on the substrate. For example, Fan et al.33 pretreated an alumina tube as a substrate, and the surface of the modified alumina tube was rich in amino groups. After a solvothermal treatment, 1,3,5-benzenetricarboxaldehyde (TFB) and p-phenylenediamine (Pa) polymerized on the surface of the alumina tubes to form a continuous COF–LZU1 membrane (Fig. 3b). As the research on COF membranes continues, not only are COF membranes prepared by the general solvothermal method, but also some researchers have prepared bilayer COF membranes by variable temperature solvothermal methods. For example, Fan et al.34 used variable-temperature solvothermal methods to grow COF–LZU1–ACOF-1 bilayers on aminated alumina discs (Fig. 3c). The reaction solution was supplemented with TFB as the aldehyde monomer and Pa and hydrazine (Hz) as the two amino monomers. Since these two amino monomers react with TFB at different temperatures, the growth of different COF can be well controlled by changing the temperature. Because the same aldehyde-based monomer is used for the polymerization of bilayer COF membranes, the two membranes COF–LZU-1 and ACOF-1 can be grown in close proximity. Besides the above COF membranes synthesized by the solvothermal method from aldehyde and amino monomers, polyimide COF membranes have also been synthesized by the condensation of amino monomers with dianhydrides. For example, Sun et al.35 used 4,4′,4′′-triamino triphenylamine (TAPA) and naphthalene-1,4,5,8-tetracarboxylic acid dianhydride (NTCAD) to polymerize COF membranes on indium–tin oxide glass substrates by a solvothermal method.
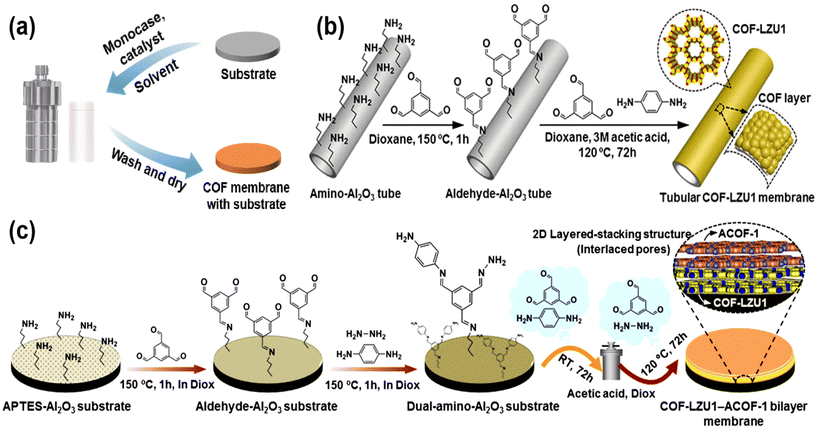 | ||
| Fig. 3 (a) The general process of preparing COF membranes by the solvothermal method. (b) Synthesis of tubular COF–LZU1 membranes. Reproduced with permission from ref. 33. Copyright 2018, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (c) Schematic representation of the synthesis of a COF–LZU1–ACOF-1 bilayer membrane by a temperature-swing solvothermal approach. Reproduced with permission from ref. 34. Copyright 2018, American Chemical Society. | ||
The solvothermal method, as a commonly available method for COF preparation, can prepare continuous COF membranes by in situ growth with the assistance of a substrate. This method is simple and easy to control. Just put the pretreated substrate into the reaction solution and react to obtain a continuous COF membrane. Moreover, it is easier to grow a dense and defect-free continuous COF membrane on the pretreated substrate. However, there are still some problems with the solvothermal method. For example, the solvothermal method usually needs to be reacted under high temperature and high pressure, which consumes a lot of energy. In addition, there are some safety hazards in the high-temperature and high-pressure reaction process. On the other hand, the capacity of the reactor limits the large-scale fabrication of COF membranes. Therefore, the large-scale fabrication of COF membranes is still a challenge. Not only that, the continuous COF membranes prepared by the solvothermal method are stacked by the interaction between COF, which determines that the COF membranes prepared by this method are usually not flexible and brittle. This also limits the use of COF membranes prepared by the solvothermal method to some extent. Although there are some drawbacks in the preparation of continuous COF membranes by the solvothermal method, researchers can choose it according to the practical needs. The solvothermal method is the most simple preparation method when the flexibility and area of the COF membrane are not required.
The interfacial polymerization strategy can be that the two monomers are dispersed in two separate phases, or that the two monomers are dispersed in one phase and the catalyst is dispersed in the other phase. No matter which polymerization strategy is used, the polymerization mechanism is roughly the same. The monomers at the interface of the two phases are continuously polymerized under the action of the catalyst, and the monomers are continuously replenished to the interface. Eventually, the continuous COF membrane is formed by the continuous polymerization of the two monomers at the confined interface. For example, Shevate et al.51 prepared COF membranes using the solvent-induced liquid–liquid interfacial polymerization of two monomers dispersed in two phases (Fig. 4a). The 1,3,5-benzenetricarboxaldehyde (Tp) was dispersed in toluene as an aldehyde-based monomer, and 2,4-pyridinediamine (Pa–Py) monomer and p-toluenesulfonic acid (pTSA) catalyst were dispersed in water. The β-ketoenamine bond-linked COF membranes were formed by interfacial polymerization. Liu et al.12 used the strategy of two monomers dispersed in one phase and catalyst dispersed in the other phase to prepare continuous COF membranes. Self-supporting flexible COF nano-membranes were prepared by a liquid–liquid interfacial reaction with TAPA and 1,3,5-triformylphloroglucinol (TFP) solutions as organic phases and acetic acid as catalyst added to the aqueous phase at room temperature and pressure (Fig. 4b). Also Liu49et al. dissolved TFP and TAPA in dichloromethane as the organic phase and pTSA as the catalyst in water for liquid/liquid interfacial polymerization. Of course, there are rare air–water interfacial synthesis methods to prepare free-standing COF membranes. For example, Pantano52et al. dissolved PDA in the aqueous phase and polymerized trace amounts of 1,3,5-tris(4-aminobenzene) benzene (TABB) with homotrimethylbenzene and a solution of 1,4-dioxane spread on the aqueous phase. Although the Pantano process for preparing COF membranes uses a trace amount of organic phase, which is thought to be the interface between air and water to form COF membranes, it is also essentially a liquid/liquid interfacial polymerization. In addition to liquid/liquid interfacial polymerization, the vapor/liquid interfacial polymerization method in interfacial polymerization is also much used in the preparation of COF membranes. For example, Yang et al.53 successfully synthesized a 1,3,5-tricarbonylresorcinol/hydrazine hydrate (TpHZ) COF layer on top of a 1,3,5-tricarbonylresorcinol/3,3-dihydroxybenzidine CTpDHBD COF layer via a thermodynamically controlled vapor/liquid interface. The Tp and n-octanoic acid catalysts were dispersed in homotrimethylbenzene as the liquid phase and poured on the surface of the CTpDHBD COF layer. The hydrazine hydrate (HZ) was dissolved in ethanol and placed underneath the CTpDHBD COF layer, and at a temperature of 30 °C, the Hz would evaporate with the ethanol and come into contact with the liquid phase above it, resulting in gas/liquid polymerization. Similarly also, Zhang et al.54 dissolved Tp in homotrimethylbenzene as the liquid phase, mixed HZ and ethanol as the gas phase (maintained at 30 °C), and sealed it for 72 h for COF growth (shown in Fig. 4c) to obtain a continuous COF membrane. In addition to the liquid/liquid and vapor/liquid interfaces mentioned above, interfacial polymerization also includes solid/liquid interfaces. For example, Wang et al.18 used electrochemical interfacial polymerization, where monomers were polymerized to form a membrane at the liquid/solid interface on the surface of a confined electrode under the action of an applied voltage (as shown in Fig. 4d). Under the condition of an applied voltage, the surface of one end electrode is charged, and the monomers in the liquid phase are continuously replenished to the solid/liquid interface, which promotes the polymerization of COF membrane.
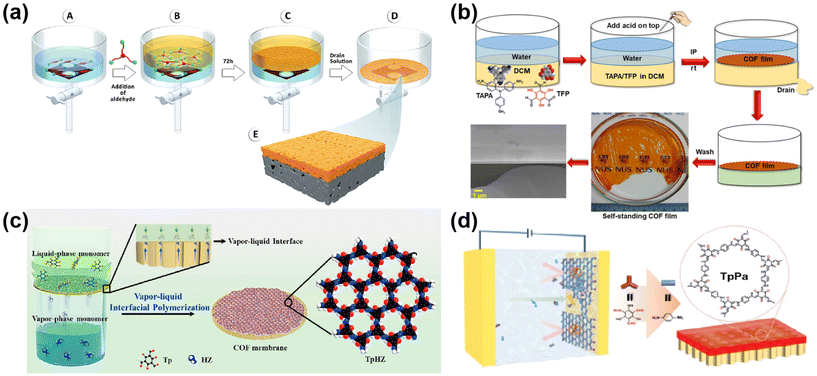 | ||
| Fig. 4 (a) Fabrication of COF membrane by 2D COF membrane self-assembly and deposition on porous supports. Reproduced with permission from ref. 51. Copyright 2022, the Authors, published by the American Chemical Society. (b) The preparation of a continuous self-standing COF membrane by a mild interfacial reaction. Reproduced with permission from ref. 12. Copyright 2020, the Authors reserve some rights; exclusive licensee American Association for the Advancement of Science. (c) The fabrication of TpHZ/PAN membranes by vapor–liquid interfacial polymerization. Reproduced with permission from ref. 54. Copyright 2021, Elsevier B.V. (d) The preparation of continuous COF membranes by electrochemical solid–liquid interfacial polymerization. Reproduced with permission from ref. 18. Copyright 2023, Wiley-VCH GmbH. | ||
In summary, the interfacial polymerization reaction conditions are relatively mild. Interfacial polymerization can prepare self-supporting membranes at room temperature and pressure. Compared with the solvothermal method to prepare COF membranes, the energy consumption is low. In addition, the interfacial polymerization method can be used to prepare COF membranes of different thicknesses by changing the addition amount of active monomer. What's more, the morphology and crystallinity as well as the physicochemical properties of COF membranes can be adjusted by changing the conditions such as the reaction time and solvent. Whether it is a liquid–liquid interface, vapor–liquid interface or solid–liquid interface, the thickness of the film can be controlled by controlling the monomer concentration. And the COF membranes grown under interfacial confinement conditions have the advantage of fewer defects. Due to the effect of interfacial confinement, most of the COF membranes prepared by interfacial polymerization are monolayer or few-layer transversely arranged COF nanosheet membranes. Therefore, the COF membranes prepared by interfacial polymerization also have a certain degree of flexibility. This can solve the problem of the lack of flexibility of COF membranes prepared by the solvothermal method mentioned above. However, compared with polymer-polymerized membranes, their mechanical properties are still somewhat deficient. The steam-assisted conversion method can be a good solution to this problem.
2.2. Top-down
The top-down preparation of COF membranes generally involves reprocessing the prepared COF powder or COF nanosheets to form a continuous COF membrane. Top-down includes the steam-assisted conversion method and layer by layer.Some researchers will use the first method to prepare COF hybrid matrix membranes. For example, Di et al.63 dispersed the prepared Fe3O4@TpBD microspheres in (N,N-dimethylacetamide) DMAc, then added the porogenic agent, and mixed it well to form a casting solution, which was cast on a glass plate and evaporated to form a membrane (Fig. 5a). There are also some researchers who use steam-assisted conditions for COF polymerization. For example, Lv et al.59 used in situ steam-assisted self-polymerization to produce COF membranes (Fig. 5b), which were converted from monomers to highly crystalline COF membranes under steam-assisted conditions, a method that allowed for the growth of membranes on a wide range of substrates and the control of the membrane thickness by varying the concentration of the monomers. Hao64et al. alternately evaporated p-phenylenediamine powders and TFB solutions and poured them onto polyacrylonitrile (PAN) membranes prepared as a COF–LZU-1 membrane, and studied the effect on the COF membrane by controlling the evaporation temperature and time. The membranes mentioned in the above examples are mostly flexible; however, with the development of COF membranes, the vapor-assisted method is not limited to the two commonly used membrane formation mechanisms mentioned above. As shown in Fig. 5c, in recent years, some researchers have prepared the monomers for COF membranes as dense blocks, and then assisted the polymerization with the help of catalyst steam to form continuous dense COF membranes.65 Since the COF membranes are dense blocks of monomers catalyzed and polymerized to form membranes with catalytic steam, the COF membranes have high strength and are flexible. This breaks the inherent perception of the steam-assisted preparation of flexible COF membranes, and dense, non-flexible pure COF membranes can also be prepared by this method.
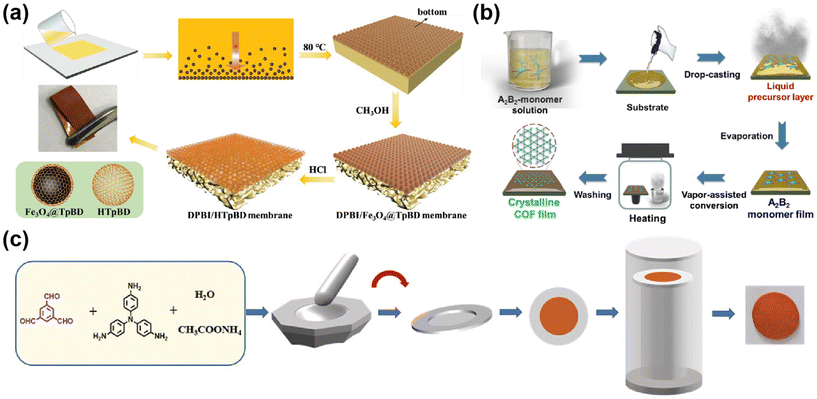 | ||
| Fig. 5 (a) The proposed preparation process of DPBI/HTpBD composite membranes. Reproduced with permission from ref. 63. Copyright 2022, Wiley-VCH GmbH. (b) The preparation of crystalline COF film by the vapor-assisted conversion method. Reproduced with permission from ref. 59. Copyright 2021, Chinese Chemical Society. (c) Reproduced with permission from ref. 65. Copyright 2023, Wiley-VCH GmbH. | ||
The steam-assisted method was originally used mostly for the preparation of polymer membranes. As COF came into the researchers’ view, some steam-assisted preparation of COF-based MMMs research appeared. This steam-assisted preparation of COF-based MMMs is flexible, which broadens the application scenario of COF membranes. However, the rapid development of COF membranes in recent years, which means the steam-assisted preparation of COF membranes is no longer limited only to mixed matrix membranes, and the steam-assisted method of various free-standing/pure COF membranes has also been widely studied. This method can not only prepare free-standing/pure COF membranes with flexibility, make up for the brittleness problem of carbon fiber membranes, and greatly improve the mechanical properties of membranes. What's more, the size of COF membranes prepared by this method can be varied according to the size of the membrane-forming mold, thus realizing the large-scale preparation of COF membranes.
Sun21 and colleagues prepared TpHz COF by interfacial polymerization, and then vacuum-assisted assembly of the prepared COF nanofibers into membranes (Fig. 6a) was achieved by layer by layer stacking on a support substrate. And TpHz COF membranes showed superior oil–water separation properties as the thickness of the membranes was regulated. Wang71et al. developed a polyelectrolyte-mediated assembly strategy. Utilizing the electrostatic interactions between polyethyleneimine and TpPa–SO3H nanosheets, 8 nm thick ultrathin COF membranes were prepared with vacuum assistance (Fig. 6b).
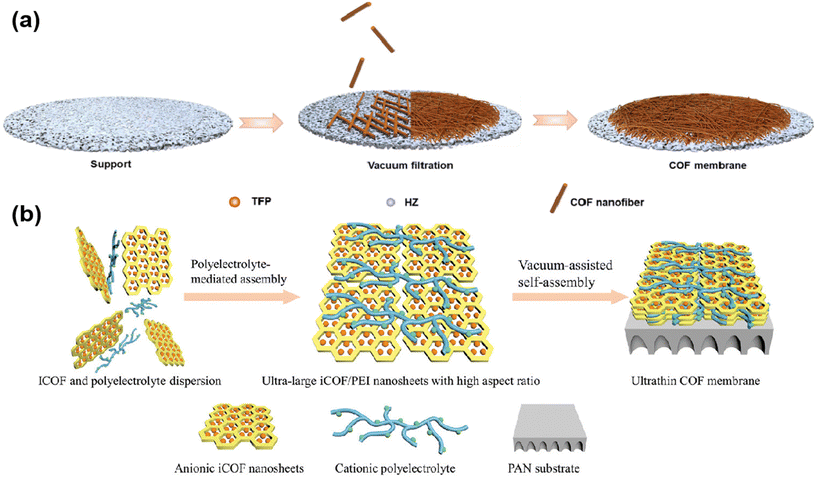 | ||
| Fig. 6 (a) The formation process of the TpHZ COF membrane. Reproduced with permission from ref. 21. Copyright 2023, Elsevier B.V. (b) Illustration of a polyelectrolyte-mediated assembly strategy to prepare ultrathin COF membranes. Reproduced with permission from ref. 71. Copyright 2023, Wiley-VCH GmbH. | ||
The advantage of this method is that the membrane thickness can be adjusted by adjusting the concentration of COF in the dispersion. This method allows for the preparation of ultra-thin membranes, the thinner the better for reducing the resistance and time for molecules to pass through the membrane, resulting in rapid separation.
3. The properties of COF membranes
In the field of membrane separation technology, the two keys to evaluate the performance of membrane separation are separation flux and separation efficiency. However, the separation flux and separation efficiency depend on the properties of the membrane (aperture, hydrophilicity/hydrophobicity and surface charge). Aperture regulation is the key to realize membrane separation. Nevertheless, it is difficult to regulate only the aperture of the membrane to meet the requirements of separation flux and separation efficiency in practical applications. In addition to the regulation of the aperture of the membrane, the regulation of the surface wettability and surface chargeability of the membrane can help to further realize the improvement of separation flux and separation efficiency.3.1. Aperture
The aperture is one of the important properties of membrane materials. Conventional polymer membranes such as polyamide membranes have fewer pores or rely on porogenic agents to form pores and have uncontrolled pore size tuning, which limits their applications.72,73 However, the natural pore structure and aperture tunable nature of COF have made free-standing/pure COF membranes popular among researchers.The aperture of COF mainly depends on the spatial structure of the organic monomer. When the monomer contains multiple benzene ring structures, the synthesized COF will have larger apertures,74,75 while when the monomer is a single chain such as ethylenediamine, the prepared COF have smaller apertures. To further reduce the apertures of the COF, monomers with larger side groups or functional groups can be selected.80
Currently, the reported apertures of COF membranes range from 0.4 nm to 5.8 nm,76–78 in the size range of nanofiltration and ultrafiltration membranes for desalination, osmosis evaporation, water treatment and organic solvent nanofiltration. Nanofiltration membranes used for desalination usually have relatively small apertures, typically less than 1 nm. For example, Xiao79 and colleagues used the secondary growth method to design COF membranes for desalination, and the pore size of the membranes contracted from 0.96 nm to 0.71 nm after the secondary growth (Fig. 7a). Furthermore, the membranes were also denser after the secondary growth, which was conducive to the enhancement of desalination efficiency. For membranes used for the nanofiltration of organic solvents, the aperture is typically less than 2 nm. More importantly, the aperture and porosity of COF-based membranes affect their application in membrane separation. The methods of adjusting pore size include: (1) changing the length and structure of the organic monomer to affect the aperture and shape. For example, Mishra47et al. chose three diamines as monomers for the preparation of a COF and found that the COF aperture increases as the length of the diamine chain grows. (2) Introducing the larger side groups or functional groups into the crystal network.80 As shown in the figure, the inside of the COF pores is further narrowed due to the presence of groups. This approach extends the range of adjustable pore sizes beyond the limitations of the size of the organic monomers themselves (Fig. 7b). (3) Using organic monomers with multiple attachment sites for the reaction to construct double or triple wells as a way to improve the selectivity of the aperture. For example, Chen81 and colleagues used hexagonal symmetric monomers as tetra-connected units with tetra-connected monomers to synthesize topologically structured three-dimensional COF. Due to the partial covalent attachment of the aldehyde monomers, a portion of the benzaldehyde is retained in the COF, which facilitates the gas adsorption and separation of three-dimensional COF (Fig. 7c).
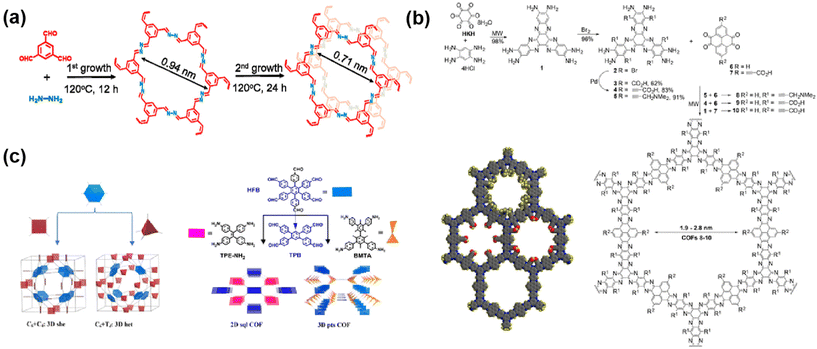 | ||
| Fig. 7 (a) Aperture changes in COF after primary and secondary growth. Reproduced with permission from ref. 79. Copyright 2021, Elsevier. (b) Synthesis process and pore size of COF 8–10. Reproduced with permission from ref. 82. Copyright 2018, American Chemical Society. (c) Design and synthesis of three-dimensional mesh COF. Reproduced with permission from ref. 81. Copyright 2021, American Chemical Society. | ||
In the field of membrane separation technology, the aperture of the membranes is critical. This not only determines the flux (separation rate) during the membrane separation process, which likewise governs the separation efficiency of the membrane. Larger apertures indicate higher fluxes, which also means that the separation process is not pure and does not achieve a high separation efficiency. In order to achieve higher separation efficiencies, it is often necessary to precisely regulate the aperture of the membrane, which in turn implies a lengthy (low flux) separation process. There is a trade-off between flux and efficiency, and to break through this problem, it can also be solved by regulating the wettability and chargeability of the membrane.
3.2. Hydrophilicity/hydrophobicity
Hydrophilicity is important in membrane applications such as water and solvent separation, because it allows water molecules to diffuse rapidly through the membrane, reducing surface contamination.21,83 Therefore, the preparation of membranes using hydrophilic COF is beneficial for membrane separation. For example, Guan84et al. investigated the effect of three different linking groups on the desalination performance of COF membranes. They compared the –C group, –Ad group and –Si group and found that the presence of the –Si group resulted in better hydrophilicity of the COF membranes, which is a result of the better bonding ability of the –Si group relative to the other two groups. The problem of low water permeability due to small pore size can be solved by adjusting the hydrophilicity of the membrane. In addition to desalination, hydrophilicity is advantageous in processes such as dye extraction and osmotic evaporation.Generally there are two ways to enhance the hydrophilicity of COF; one is to select organic monomers containing more –NH2, –OH, –COOH groups as the reaction material (Fig. 8a). For example, Xu85et al. used tricarbonyl chloride and p-phenylenediamine as monomers to prepare COF containing abundant –COOH groups, resulting in membranes exhibiting enhanced hydrophilicity. The second is the introduction of groups such as –NH2, –OH, and –COOH in COF to enhance hydrophilicity through post-modification methods (Fig. 8b). For example, Han86et al. utilized ionic liquids to modify COF-367 nanosheets, and a large number of –OH groups were introduced on the COF nanosheets to enhance the hydrophilicity of the composite membrane. Lin87et al. fabricated the sulfonated COF into a hybrid matrix membrane, and the abundant –SO3 groups on the COF improved the hydrophilicity of the membrane. Also, He88et al. modified the –SO3 group by post-treatment of 1,3-propanesulfonolactone to enhance the hydrophilicity of the membrane. Because most COF are the imine group type themselves, organic monomers with –NH2 and –CHO, most COF are hydrophilic.86,89 If you want to achieve hydrophobicity, you can use the same method, using monomers containing hydrophobic groups or post-modification treatments.91,92 For example, Mohammed90 and colleagues prepared 3D COF membranes using an interfacial polymerization method at room temperature. The network defects exposed Br atoms, playing a role in enhancing the hydrophobicity of the membrane, which can selectively pass through the oil phase in the emulsion and play a role in separating oil and water. Chen91 and colleagues were synthesizing COF using fluorine-containing monomers to achieve the hydrophobicity of COF. And Yang92et al. prepared sea urchin-like COF membranes with superhydrophobicity for the gravity-driven separation of surfactant-stabilized water-in-oil emulsions.
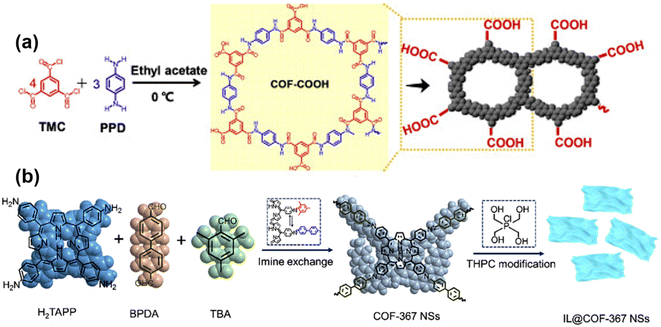 | ||
| Fig. 8 (a) Schematic illustration of the preparation of COF–COOH membrane. Reproduced with permission from ref. 85. Copyright 2020, published by Elsevier B.V. (b) Diagrammatic drawing of the synthetic procedure for the COF-367 and IL@COF-367 NSs. Reproduced with permission from ref. 86. Copyright 2022, Elsevier B.V. All rights reserved. | ||
The wettability of COF membranes can be adjusted by changing the monomer or by modification treatments after membrane formation. During the separation process, the special wettability of the COF membrane has selective permeability, which helps to improve the separation flux and separation efficiency of the membrane. Wettable COF membranes are especially advantageous for oil–water separation and desalination-related applications.
3.3. Surface charge
In addition to the above properties, the surface charge of COF also plays an important role in seawater desalination, dye extraction, and nanofiltration of organic solvents. In the separation process, due to the electrostatic repulsion between the membrane surface and the solute, the membrane with the same positive/negative charge as the solute tends to have a high retention rate and good anti-fouling performance.93 For example, Banjerdteerakul70et al. deposited the COF enriched with –COOH on a PAN membrane, resulting in a heavily negatively charged surface of the composite membrane. This membrane was used for the separation of bovine serum proteins that were also negatively charged. The cumulative effect of electrostatic repulsion as well as pore size increased the separation efficiency from 3.5% to 81.9%. Moreover, the electrostatic interaction between the COF membrane and the solute can also be utilized to adsorb organics with opposite electronegativity. Chen80 and colleagues utilized charge-controlled molecular sieving to design sulfonic acid-based COF membranes with negatively charged surfaces. The membrane can be used for cationic organic removal with cationic molecular recoveries of >99%. In the same way, Chen94et al. achieved the in situ growth of imine-based COF on anodic alumina, where the binding of imine groups to protons makes the COF positively charged, anion-selective and permeable, and enables ion transport.When the surface charge of the COF membrane is the same as that of the solute, due to electrostatic repulsion, the membrane plays a repulsive and antifouling effect on the solute, and the filtration process consists of a high retention rate. When the surface charge of the COF membrane is different from that of the solute, due to electrostatic attraction, the membrane acts as an adsorbent to the solute, and can be used for the adsorption and recovery of dyestuffs, salts, and so on. Therefore, the surface charge of the membrane can be appropriately regulated according to the different application requirements.
4. The applications of COF membranes
COF membranes are very promising in the field of membrane separation technology. Due to the COF membranes’ tunable pore size, wettability and electronegativity, the membranes have been widely used in gas separation, water treatment, organic solvent nanofiltration and pervaporation.4.1. Gas separation
The amorphous polymers prepare conventional membranes with disordered and inconsistent apertures.15 It is difficult to achieve beyond the current Robeson upper limit. COF with abundant and well-organized in-plane pores are particularly promising for achieving ultrafast and highly selective molecular sieving.97 In particular, COF have excellent chemical and physical stability as well as tunable pore sizes. As energy and environmental problems become more and more serious, COF are increasingly used to separate gases such as H2, CH4, CO2, etc.98–101In practical applications researchers have developed several types of COF-based membrane for gas separation. Early separation membranes were formed by combining COF and a substrate to form a composite membrane, with the substrate providing support for the continuous membrane formation of the COF.34 In order to precisely regulate the pore size, researchers are constantly exploring new methods. And COF, as a covalent organic framework material with natural porosity as well as pore-channel tunable properties, began to enter the vision of researchers. Gas separation membrane materials are not limited to polymer membranes. At the same time, pure COF membranes are gradually emphasizing the advantages of pore size, and are rapidly developing in the field of gas separation. For example, Fan95 and colleagues chose alumina as the substrate to grow the CoAl-layered double hydroxide layer as a domain-limited template. Stabilized imide-based COF–LZU1 (the condensation of 1,3,5-triformylbenzene with para-phenylenediamine) was selected for in situ growth on the treated substrate to obtain vertically aligned COF membranes (Fig. 9a). The vertically aligned membrane structure allows for higher fluxes in the gas separation process. With a high H2 permeability of 3600 GPU, the COF–LZU1 membrane provides ideal separation selectivity for gas mixtures such as H2/CO2 (31.6) and H2/CH4 (29.5). In addition to the basic pure continuous COF membranes, researchers continue to innovate and discover new methods in the pore size regulation of COF membranes. For example, the concept of “pore-in-pore” has been proposed by reconstructing pores inside the COF pores. A similar separation effect can be achieved through the “pore-in-pore” mode. For example, Fan96 and colleagues used the pore-within-a-pore concept to assemble linear α-cyclodextrin (α-CD) in the hollow pore channels of COF. The α-CD-in-COF membranes have ultramicroporous nanopore channels that exhibit high selectivity (>30) and permeability (3000 GPU) to H2 (Fig. 9b).
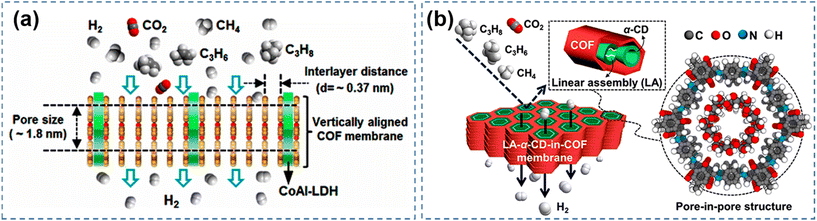 | ||
| Fig. 9 (a) Gas separation mechanism of vertically aligned COF membranes. Reproduced with permission from ref. 95. Copyright 2020, American Chemical Society. (b) Gas separation mechanism of the pore-in-pore structure α-CD COF membranes. Reproduced with permission from ref. 96. Copyright 2023, the Authors. Published by the American Chemical Society. | ||
The two most important criteria for gas separation performance are the separation factor and permeability. Some applications of COF membranes in gas separation are listed in Table 1. As demonstrated in Table 1, a comparison of the gas separation performance shows that a relatively high separation factor is associated with a relatively low gas permeability. However, this conclusion is not absolute, because more and more COF membranes can break through the Robeson upper limit by pore size modulation. However, the most critical determinant of COF membrane separation performance is the pore size. The higher the separation factor, the higher the pore size requirement of the membrane, which needs to be precisely regulated.102 The pore size of the gas membrane has to be between the size of the molecular diameters of the two separated gases, with a smaller pore size. In this case, there is a problem of relatively lower permeability. At this point, researchers need to make trade-offs between the separation factor and permeability of the membrane based on application scenarios, application conditions, and so on.
| Membrane materials | Gas separation | Membrane thickness (nm) | Temperature (K) | Pressure (bar) | Separation Factor | Permeance (GPU) | Ref. |
|---|---|---|---|---|---|---|---|
| iCOF@PEI | CO2/N2 | 8 | 298 | 2 | 33 | 1371 | 71 |
| TpTGCl@TpPa–SO3H | H2/CO2 | 155 | 423 | — | 26 | 2163 | 103 |
| COF–LZU1 | H2/CO2, H2/CH4 | ∼2000 | 298 | 1 | 31.6, 29.5 | ∼3600 | 95 |
| EBAM-2 | C3H6/C3H8 | 320 | 298 | 1.1 | 203 | 168 | 104 |
| LA-α-CD-in-TpPa-1 | H2/CO2, H2/CH4 | ∼1500 | 298 | 1 | >30 | ∼3000 | 96 |
| ZIF-67-in-TpPa-1 | H2/CO2, H2/CH4 | ∼1000 | 298 | 1 | 34.9, 33.3 | >3000 | 105 |
| N–COF | H2/CO2 | ∼800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
298 | 1.1 | 13.8 | 4319 | 65 |
| KUF-3 | C2H6/C2H4 | — | 298 | 1 | 1.97 | — | 106 |
| NCOFM-50 | CO2/N2, CO2/CH4 | 200 | 298 | 2 | 80, 54 | >300 | 107 |
4.2. Water treatment
The membrane separation has proved to be a safe, energy-efficient and environmentally sustainable method to meet the growing demand for clean water, hence its wide application in desalination and wastewater recycling.3,109 Meanwhile, most of the reported COF pore sizes are in the range of 0.4 nm–5.8 nm. In this range, the membrane is suitable for the nanofiltration and ultrafiltration of salts, dyes, and other organics in water. In the field of water treatment applications, whether it is the removal of dyes and salts from water or the separation of oil and water, it is the wettability of the membrane that plays a decisive role. The wettability of the COF membrane determines the flux size of the membrane. Second, the pore size of the COF membrane determines the membrane separation efficiency, since some salts, dyes, microemulsion droplets stabilized by surfactants, etc. are common in water treatment applications. Therefore, the surface charge of the COF membrane also plays a role.For example, Liu108 and colleagues prepared continuous flexible ACOF-1 (hydrazine hydrate/1,3,5-triformylbenzene condensed) membranes on hydrolyzed polyacrylonitrile (HPAN) substrates using interfacial polymerization (Fig. 10a). By adjusting the hydrazine hydrate concentration for COF membrane optimization, the optimized ACOF-1/HPAN membranes showed an ultra-high water permeability of 142 L per m2 per h per bar and contaminant retention greater than 65%. High hydrophilicity of the membranes results in high flux during the separation process. There are also some similar studies on growing COF on polymer substrates for water treatment. For example, Su110et al. made COF–LZU1/PES composite membranes by the in situ interfacial polymerization of COF–LZU1 layers on polyethersulfone (PES) microfiltration membranes with an average pore size of 0.2 μm. While the membrane thickness gradually increased with the increase of reaction time, the purified water permeability of COF–LZU1/PES composite membranes decreased from 328 L per m2 per h per bar to 55.8 L per m2 per h per bar. Meanwhile, the rejection rate of chromium black-T gradually increased. This phenomenon is similar to the relationship between the separation factor and permeability mentioned above in gas separation. In addition to tuning and optimizing the pore size and thickness of the membrane for better performance, imparting electronegativity to the membrane and utilizing electrostatic interactions to improve membrane performance is also an effective method. For example, Basel93 and colleagues performed liquid–liquid interfacial polymerization based on previous studies. The defect-free triformylphloroglucinol ethidium bromide (TpEB) COF membranes were formed on the carrier surface. It was found that cationic TpEB COF composite membranes have >98% retention for a wide range of anionic dyes, independent of the size of the dye molecule, due to the positive charge on the surface of the pore. As well, Yang48 and colleagues prepared an anionic TpPa–SO3Na (1,3,5-triformylphloroglucinol and 2,5-diaminobenzenesulfonic acid condensed) COF membrane and analyzed the dye separation mechanism (Fig. 10b). It is considered that the cationic dyes are adsorbed and immobilized by the negatively charged COF pores, and the anionic dyes will move away from the membrane surface due to electrostatic repulsion. The Table 2 lists some applications of COF membranes in dye removal and salt removal.
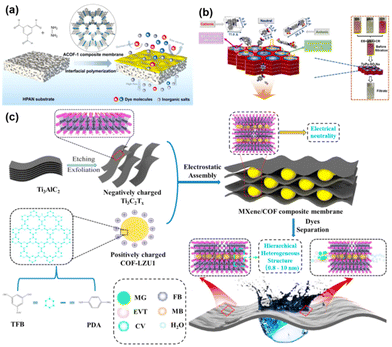 | ||
| Fig. 10 (a) Scheme of the fabrication of the ACOF-1/HPAN composite membranes. Reproduced with permission from ref. 108. Copyright 2021, American Chemical Society. (b) Schematic illustration of the mechanism for selective dye molecule separation through the negatively charged TpPa–SO3Na membrane. Reproduced with permission from ref. 48. Copyright 2022, Elsevier B.V. All rights reserved. (c) Schematic demonstration of the fabrication of MXene/COF composite membrane and its molecular sieving mechanism. Reproduced with permission from ref. 69. Copyright 2022, Elsevier B.V. All rights reserved. | ||
| Membrane materials | Membrane thickness (nm) | Salt/dye | Salt/dye concentration (ppm) | Pressure (bar) | Rejection rate (%) | Flux (L per m2 per h per bar) | Ref. |
|---|---|---|---|---|---|---|---|
| cCOF–PA-3 | 11 | MgCl2 | 1000 | 2 | 99.4 | 10.1 | 25 |
| TpPa–SO3H/TpPa–SO3H/MPAN | ∼200 | Na2SO4 | 1000 | 5 | 98.3 | 13.1 | 111 |
| BC/COF composite membrane | ∼28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Congo red | 50 | 1 | 99.37 | 194.99 | 112 |
| COF–LZU1-2d | 469 | Rose Bengal stain | 50 | 5 | 99.9 | 2.82 | 113 |
| pDA/TpPa (W/E)–COF | ∼125 | Na2SO4 | 1000 | 5 | 99.5 | 51.3 | 114 |
| CTF-1 | — | NaCl | — | — | 91 | ∼2675 | 115 |
| TpHz/PES membrane | ∼12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
Na2SO4 | 1000 | 4 | 58.3 | 4.05 | 116 |
| PaTP0.05%–TMC0.2%/30 membrane | ∼159 | NaCl | 1000 | 5 | 93.3 | 0.81 | 117 |
Unlike gas separation, membrane surfaces are wetted by water or other solvents during water treatment applications. In addition to aperture size, membrane wettability plays a critical role in the water treatment process. Similar to gas separation, there is a trade-off between the flux and separation efficiency of COF membranes, which is influenced by pore size. However, methods that impart special wettability to COF membranes can break this barrier. Appropriate hydrophilicity can result in a significant increase in water flux through the COF membrane without affecting the separation efficiency. In the process of membrane separation, the surface electronegativity of the membrane also plays a role for salt retention in water. Therefore, researchers should give full consideration to the regulation of membrane properties according to the application scenarios and application requirements in designing COF membranes for water treatment.
4.3. Organic solvent nanofiltration
The organic solvent nanofiltration membranes typically have an aperture of 1–2 nm and are commonly used for the exchange, purification and recovery of organic solvents. This field has gained much attention as a prospective separation technology in the chemical and pharmaceutical industries. In organic solvent nanofiltration separations, the membrane is immersed in an organic solvent, so stability of the membrane is particularly important. For example, imido COF, hydrazine-linked COF, and especially ketoamino COF are stable in organic solvents and are suitable to be used as materials for the preparation of organic solvent nanofiltration membranes.For example, Shi78 and colleagues designed a three-dimensional COF membrane with subnanometer antiswelling channels. Three-dimensional topological design enabled the COF membranes to have uniformly interpenetrating sub-nanometer-sized channels, which resulted in the prepared TFPM–HZ/PAN (TFPM–HZ is condensed from tetrakis(4-formylphenyl)-methane and hydrazine hydrate) membranes showing excellent separation performance (Fig. 11a). As a result, the high porosity of the membrane allowed the membrane to exhibit a methanol permeation of 44 L per m2 per h per bar. Researchers have studied not only three-dimensional COF membranes but also two-dimensional COF membranes extensively. For example, Yin118 and colleagues prepared COF polyamide composite membranes by in situ interfacial polymerization. In contrast to the pristine membrane, the introduction of COF increased the membrane aperture to 0.02 nm. The ethanol permeability of the composite membrane with optimized monomer ratios was 65.7 L m−2 h−1 MPa−1, and the retention of rhodamine-B was 99.0% (Fig. 11b). And Zhang114et al. developed an in situ molecular welding strategy to prepare defect-free COF membranes. The dopamine was self-polymerized in an alkaline environment, and the resulting polydopamine acted as a “solder” to weld the COF in situ (Fig. 11c). This strategy not only improves membrane stability but also reduces the separation size of the membrane. This study prepared pDA/TpPa (W/E)–COF membranes with permeation rates of more than 86 L per m2 per h per bar to ethanol, methanol, acetone, hexane, and acetonitrile. At the same time, the retention rate for a variety of dyes is more than 95%.
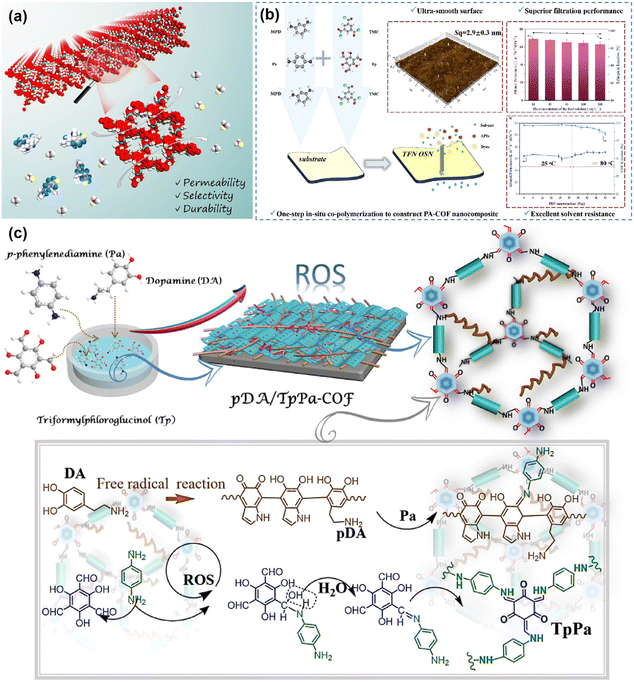 | ||
| Fig. 11 (a) Three-dimensional COF membrane organic solvent nanofiltration process. Reproduced with permission from ref. 78. Copyright 2022, Wiley-VCH GmbH. (b) Introduction on TNF nanofiltration membrane preparation and performance. Reproduced with permission from ref. 118. Copyright 2023, Elsevier B.V. All rights reserved. (c) The process of in situ molecular soldering engineering to fabricate COF membranes. Reproduced with permission from ref. 114. Copyright 2021, the Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. | ||
For the application of COF membranes in organic solvent nanofiltration, the aperture of the membrane is still critical since the membrane will be immersed in the organic solvent for a long time during the organic solvent nanofiltration process. Therefore, in the design process of organic solvent nanofiltration membranes, in addition to the aperture, wettability and electronegativity, the stability of membrane also needs to be considered.
4.4. Pervaporation
Pervaporation is an energy efficient and promising technology for liquid separation in refineries, and the petrochemical and pharmaceutical industries. In pervaporation, the membrane first absorbs the components of the mixture and then diffuses the mixture through a gradient of chemical potential caused by vacuum or gas purification. Ultimately, the mixture is separated by the adsorption and diffusion behavior in pervaporation. The application of pervaporation in separation can be categorized into three main areas: (1) the dehydration of aqueous–organic mixtures, (2) the separation of trace volatile organic compounds from aqueous solutions, and (3) the separation of organic–organic solvent mixtures.Due to its good compatibility and versatility, COF are introduced into polymer membranes to enhance or hinder the adsorption and diffusion of components during the pervaporation process. Therefore, COF-based polymer membranes have excellent selectivity and high permeability. With the rapid development of hydrophilic COF, hydrophilic COF-based mixed matrix membranes have been widely used in pervaporation, mainly focusing on ethanol dehydration and butanol dehydration. For example, Cao119et al. used the post-synthetic linker exchange method to facilely synthesize polyacrylonitrile-based COF membranes (TpPa@Hz) with the introduction of –NH2. This process enhances the hydrophilicity of the membranes and facilitates the selectivity of the alcohol dehydration process. In C4 alcohol/water separation, the flux of the TpPa@Hz (4) membranes was greater than 5 kg m−2 h−1 and the separation factor of the TpPa@Hz (4) membranes was greater than 3000. Furthermore, Yang53et al. interfacially synthesized a TpHZ@CTpDHBD membrane for water/n-butanol separation with an ultra-high separation coefficient of 4464 and an ultra-high permeate flux of 14.35 kg m−2 h−1. This is due to the fact that the aperture of COF is around 0.39 nm, whereas the n-butanol molecule size is around 0.51 nm and it is difficult for it to pass through the pores. The COF membrane acted with a molecular sieving effect (Fig. 12a). Zhang et al.54 prepared TpHZ/PAN membranes to achieve an ultra-high water flux of 8.16 kg m−2 h−1 with a n-butanol dehydration separation factor of 1023. Not only that, but there are studies such as those by Banjerdteerakul120 and colleagues, who chose two imine-bonded COF to study the pervaporization separation of organic solvents. The yttria-stabilized zirconia hollow fibers were selected as the substrate material, and COF–LZU1 (1,3,5-triformylbenzene and p-phenylenediamine condensed) and COF-300 (tetrakis-(4-anilyl)-methane and terephthalaldehyde condensed) were prepared as membranes by in situ growth on the substrate via solvothermal method. The azeotropic mixtures of both membranes were tested for pervaporation at a constant osmotic pressure of 0.0066 atm (Fig. 12b). Among these, the flux of acetone/cyclohexane separation was more than 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g m−2 h−1.
000 g m−2 h−1.
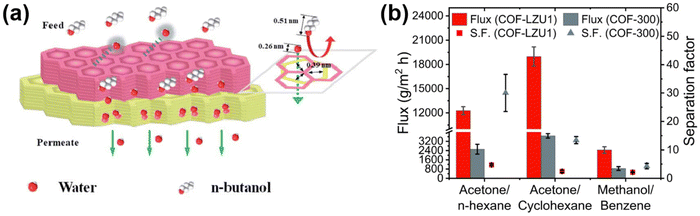 | ||
| Fig. 12 (a) Yang53et al.'s design of a bilayer COF membrane with 0.39 nm pores for the separation of water and n-butanol. Reproduced with permission from ref. 53. Copyright 2020, Royal Society of Chemistry. (b) The flux and separation factor of COF membranes (COF–LZU1 and COF-300). Reproduced with permission from ref. 120. Copyright 2023, the Authors. Published by Elsevier B.V. | ||
Among the various membrane separation technology tools, pervaporation is extremely demanding in terms of membrane aperture. Pervaporation requires a precise control of the membrane aperture size, between the separated substances. Ensure that only one solvent passes through the pore size to achieve separation. As a result, researchers can carry out the design of pervaporation COF membranes from the precise control of the membrane aperture.
5. Summary and outlook
COF, as an organic covalently linked crystalline polymer with high porosity, adjustable pore size and easy functionalization, is a strong contender in the field of membrane separation technology as an emerging material. COF-related membrane materials have attracted wide attention.(1) With the continuous research on COF membranes, the preparation methods of COF membranes are more diversified, and the application fields are also being broadened. In the process of the continuous development of COF membranes, independent/pure continuous COF membranes are continuously explored for more advantages. For example, pure COF membranes maximize the role of COF in membrane separation. The problem of low performance due to a low COF content in mixed matrix membranes is solved. Although there have been many studies on the preparation methods of COF membranes (solvothermal, interfacial polymerization, steam assisted conversion, layer by layer), there are still limitations in the membrane preparation methods. Therefore, the preparation method of COF membranes needs to be further investigated. In the future, the problem to be solved is how to prepare new COF membranes with high crystallinity, uniformity and excellent performance.
(2) COF with apertures less than 1 nm are highly in demand to construct gas separation membranes with high selectivity, synthesize nanofiltration membranes for seawater desalination with high salt retention, and prepare nanofiltration membranes for organic solvents with small molecular weights. Such COF can be prepared by using relatively small and short organic monomers, but there are limitations to this approach. The studies on the long-term stability of COF membranes under real separation conditions are still limited. In addition, the relatively high cost of COF, as well as the complex and time-consuming fabrication methods, may hinder the large-scale fabrication of COF membranes. Therefore, low-cost COF and cost-effective fabrication strategies should be developed to control the cost of COF membranes in industrial applications. The COF membranes still face challenges in practical applications in the future.
(3) The separation mechanism of COF membrane needs further in-depth study. Currently most of the membrane separation studies based on COF are based on molecular/ionic sieving by adjusting the aperture of the membrane. Alternatively, the surface charge, hydrophilicity and other properties of the membrane are modulated to obtain better separation. However, there are fewer studies on the separation mechanism of COF membranes. In the future, the structure and separation process of the membranes can be further studied from the microscopic point of view because synthesizing COF membranes that are more suitable for practical applications necessitates an in-depth study of the separation mechanism.
In summary, this paper comprehensively summarizes the research on COF membranes in recent years from three aspects: preparation methods, properties and applications. There is a focused discussion on stand-alone/pure COF membranes. A deep study of the preparation methods and properties of COF membranes helps researchers to design and prepare COF membranes according to different application scenarios and requirements. We hope that this work can provide guidance for researchers in the field of COF membranes and promote the development of COF membranes in the direction of gas separation, organic solvent nanofiltration and other applications.
Author contributions
Y. C. and Y. Y. wrote the draft of the manuscript, organized the figures, and selected the appropriate references. J. W. and J. Q. revised the manuscript. Y. C., J. H., D. T. and J. L. conceived the theme and the outline of this manuscript, directed the research program, and revised the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge financial support provided by the National Natural Science Foundation of China (22108125), Natural Science Foundation of Jiangsu Province (BK20210627), China Postdoctoral Science Foundation (2023M730484), China Postdoctoral Science Foundation (2023M730484), and Independent Innovation of Agricultural Science and Technology in Jiangsu Province (CX(21)3163).References
- A. P. Côté, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166–1170 CrossRef PubMed
.
- B. Chen, H. Xie, L. Shen, Y. Xu, M. Zhang, M. Zhou, B. Li, R. Li and H. Lin, Small, 2023, 19, 2207313 CrossRef CAS PubMed
.
- X. Qin, X. Qin, X. Xu, J. Zhao, Y. Gui, H. Guo, J. Mao, Y. Wang and Z. Zhang, Desalination, 2023, 557, 116598 CrossRef CAS
.
- P. She, Y. Qin, X. Wang and Q. Zhang, Adv. Mater., 2022, 34, 2101175 CrossRef CAS PubMed
.
- J. Sun, Y. Xu, Y. Lv, Q. Zhang and X. Zhou, CCS Chem., 2023, 5, 1259–1276 CrossRef CAS
.
- Y. Zhao, H. Gu, Y. Zhou, C. Wen, X. Liu, S. Wang, Z. Chen, H. Yang and X. Wang, J. Environ. Sci., 2023 DOI:10.1016/j.jes.2023.06.037
.
- S. Hameed, A. Hayat, E. A. Alghamdi, N. Bano, J. Farhat and A. Khurramd, J. Sci.: Adv. Mater. Devices, 2023, 8, 100632 CAS
.
- C. Fan, H. Wu, J. Guan, X. You, C. Yang, X. Wang, L. Cao, B. Shi, Q. Peng, Y. Kong, Y. Wu, N. A. Khan and Z. Jiang, Angew. Chem., Int. Ed., 2021, 60, 18051–18058 CrossRef CAS PubMed
.
- B.-J. Yao, J.-T. Li, N. Huang, J.-L. Kan, L. Qiao, L.-G. Ding, F. Li and Y.-B. Dong, ACS Appl. Mater. Interfaces, 2018, 10, 20448–20457 CrossRef CAS PubMed
.
- L. Cao, H. Wu, Y. Cao, C. Fan, R. Zhao, X. He, P. Yang, B. Shi, X. You and Z. Jiang, Adv. Mater., 2020, 32, 2005565 CrossRef PubMed
.
- G. Liu, Z. Jiang, H. Yang, C. Li, H. Wang, M. Wang, Y. Song, H. Wu and F. Pan, J. Membr. Sci., 2019, 572, 557–566 CrossRef CAS
.
- J. Liu, G. Han, D. Zhao, K. Lu, J. Gao and T.-S. Chung, Sci. Adv., 2020, 6, eabb1110 CrossRef CAS PubMed
.
- Y. Wang, J. Zhao, S. Zhang, Z. Zhang, Z. Zhu, M. Wang, B. Lyu, G. He, F. Pan and Z. Jiang, Mater. Horiz., 2023, 10, 5016–5021 RSC
.
- F. Wang, Z. Zhang, I. Shakir, C. Yu and Y. Xu, Adv. Sci., 2022, 9, 2103814 CrossRef CAS PubMed
.
- C. Wu, L. Xia, S. Xia, B. Van der Bruggen and Y. Zhao, Small, 2023, 19, 2206041 CrossRef CAS PubMed
.
- J. Ravi, M. H. D. Othman, T. Matsuura, M. R. I. Bilad, T. El-Badawy, F. Aziz, A. Ismail, M. A. Rahman and J. Jaafar, Desalination, 2020, 490, 114530 CrossRef CAS
.
- H. Feng, K. Yuan, Y. Liu, B. Luo, Q. Wu, X. Bao, W. Wang and J. Ma, Chem. Eng. J., 2023, 474, 145580 CrossRef CAS
.
- M. Wang, Y. Wang, J. Zhao, J. Zou, X. Liang, Z. Zhu, J. Zhu, H. Wang, Y. Wang, F. Pan and Z. Jiang, Angew. Chem., Int. Ed., 2023, 62, e202219084 CrossRef CAS PubMed
.
- S. Yuan, X. Li, J. Zhu, G. Zhang, P. Van Puyvelde and B. Van der Bruggen, Chem. Soc. Rev., 2019, 48, 2665–2681 RSC
.
- S. Patial, V. Soni, A. Kumar, P. Raizada, T. Ahamad, X. M. Pham, Q. V. Le, V.-H. Nguyen, S. Thakur and P. Singh, Environ. Res., 2023, 218, 114982 CrossRef CAS PubMed
.
- Q. Sun, J. Du, L. Wang, A. Yao, Z. Song, L. Liu, D. Cao, J. Ma, W. Lim, W. He, S. Ul Hassan, C. Zhou and J. Liu, Sep. Purif. Technol., 2023, 317, 123825 CrossRef CAS
.
- C. Fan, L. Cao, C. Yang, Q. Xiao, X. You, X. Wang, Y. Kong, H. Wu, Y. Liu and Z. Jiang, J. Membr. Sci., 2022, 645, 120186 CrossRef CAS
.
- L. Samineni and M. Kumar, Nat. Nanotechnol., 2022, 17, 564–566 CrossRef CAS PubMed
.
- Y. Pu, M. Zhao, X. Liang, S. Wang, H. Wang, Z. Zhu, Y. Ren, Z. Zhang, G. He, D. Zhao and Z. Jiang, Angew. Chem., Int. Ed., 2023, 62, e202302355 CrossRef CAS PubMed
.
- G. Wang, T. Wu, J. Zhao, B. Shi, T. Gu, R. Zhang, X. Wang, R. Kasher, Y. Su and Z. Jiang, J. Membr. Sci., 2023, 684, 121863 CrossRef CAS
.
- F. Kang, X. Wang, C. Chen, C.-S. Lee, Y. Han and Q. Zhang, J. Am. Chem. Soc., 2023, 145, 15465–15472 CrossRef CAS PubMed
.
- X.-H. Han, J.-Q. Chu, W.-Z. Wang, Q.-Y. Qi and X. Zhao, Chin. Chem. Lett., 2022, 33, 2464–2468 CrossRef CAS
.
- S. Kandambeth, J. Jia, H. Wu, V. S. Kale, P. T. Parvatkar, J. Czaban-Jóźwiak, S. Zhou, X. Xu, Z. O. Ameur, E. Abou-Hamad, A.-H. Emwas, O. Shekhah, H. N. Alshareef and M. Eddaoudi, Adv. Energy Mater., 2020, 10, 2001673 CrossRef CAS
.
- C. Qian, Q.-Y. Qi, G.-F. Jiang, F.-Z. Cui, Y. Tian and X. Zhao, J. Am. Chem. Soc., 2017, 139, 6736–6743 CrossRef CAS PubMed
.
- Y. Zhi, P. Shao, X. Feng, H. Xia, Y. Zhang, Z. Shi, Y. Mu and X. Liu, J. Mater. Chem. A, 2018, 6, 374–382 RSC
.
- J. W. Colson, A. R. Woll, A. Mukherjee, M. P. Levendorf, E. L. Spitler, V. B. Shields, M. G. Spencer, J. Park and W. R. Dichtel, Science, 2011, 332, 228–231 CrossRef CAS PubMed
.
- X. Shi, Z. Zhang, S. Fang, J. Wang, Y. Zhang and Y. Wang, Nano Lett., 2021, 21, 8355–8362 CrossRef CAS PubMed
.
- H. Fan, J. Gu, H. Meng, A. Knebel and J. Caro, Angew. Chem., Int. Ed., 2018, 57, 4083–4087 CrossRef CAS PubMed
.
- H. Fan, A. Mundstock, A. Feldhoff, A. Knebel, J. Gu, H. Meng and J. Caro, J. Am. Chem. Soc., 2018, 140, 10094–10098 CrossRef CAS PubMed
.
- B. Sun, X. Li, T. Feng, S. Cai, T. Chen, C. Zhu, J. Zhang, D. Wang and Y. Liu, ACS Appl. Mater. Interfaces, 2020, 12, 51837–51845 CrossRef CAS PubMed
.
- J. Wu, J. Gao, S.-B. Chen and T.-S. Chung, Chem. Eng. J., 2023, 469, 143982 CrossRef CAS
.
- J. Chen, J. Zhang, X. Wu, X. Cui, W. Li, H. Zhang, J. Wang, X.-Z. Cao and P. Zhang, J. Mater. Chem. A, 2020, 8, 9160–9167 RSC
.
- S. Hussain, S. Bahadar, G. Wang, L. Zhu, Z. Ye and X. Peng, Chem. Eng. J., 2022, 440, 136012 CrossRef CAS
.
- W. Lai, L. Liu, J. Bai, L. Xiao, Y. Jiao, Y. Yang, L. Shan and S. Luo, Chem. Eng. J., 2023, 460, 141708 CrossRef CAS
.
- Q. Gan, L. E. Peng, H. Guo, Z. Yang and C. Y. Tang, Environ. Sci. Technol., 2022, 56, 10308–10316 CrossRef CAS PubMed
.
- Q. Peng, Y. Lu, W. Fang, Y. Zhu and J. Jin, Chem. Eng. J., 2023, 471, 144706 CrossRef CAS
.
- C. A. C. Chazot, C. J. Thrasher, A. Peraire-Bueno, M. N. Durso, R. J. Macfarlane and A. J. Hart, Adv. Funct. Mater., 2023, 33, 2214566 CrossRef CAS
.
- M. I. Baig, P. G. Ingole, J.-D. Jeon, S. U. Hong, W. K. Choi and H. K. Lee, Chem. Eng. J., 2019, 373, 1190–1202 CrossRef CAS
.
- X. Jing, X. Hu, Q. Zhang, P. Feng, S. Li, Y. Liu and H.-Y. Mi, Carbon, 2023, 206, 286–294 CrossRef CAS
.
- M. Matsumoto, L. Valentino, G. M. Stiehl, H. B. Balch, A. R. Corcos, F. Wang, D. C. Ralph, B. J. Mariñas and W. R. Dichtel, Chem, 2018, 4, 308–317 CAS
.
- T. Hosokawa, M. Tsuji, K. Tsuchida, K. Iwase, T. Harada, S. Nakanishi and K. Kamiya, J. Mater. Chem. A, 2021, 9, 11073–11080 RSC
.
- B. Mishra and B. P. Tripathi, J. Mater. Chem. A, 2023, 11, 16321–16333 RSC
.
- Y. Yang, G. Li, D. Ouyang, Z. Cai and Z. Lin, Chem. Eng. J., 2023, 456, 141008 CrossRef CAS
.
- Y. Liu, Y. Gu, J. Bao, F. Liu, H. Xie and W. Wu, J. Alloys Compd., 2023, 963, 171138 CrossRef CAS
.
- Y. Li, Q. Wu, X. Guo, M. Zhang, B. Chen, G. Wei, X. Li, X. Li, S. Li and L. Ma, Nat. Commun., 2020, 11, 599 CrossRef CAS PubMed
.
- R. Shevate and D. L. Shaffer, ACS Nano, 2022, 16, 2407–2418 CrossRef CAS PubMed
.
- M. F. Pantano, E. Missale, L. Gazzato, R. Pilot, F. Sedona, G. Speranza and M. Frasconi, Mater. Today Chem., 2022, 26, 101007 CrossRef CAS
.
- H. Yang, H. Wu, Y. Zhao, M. Wang, Y. Song, X. Cheng, H. Wang, X. Cao, F. Pan and Z. Jiang, J. Mater. Chem. A, 2020, 8, 19328–19336 RSC
.
- Z. Zhang, H. Yang, C. Cao, Y. Liu, S. Liang, M. Wang, H. Wang, X. Cao, F. Pan and H. Wu, J. Membr. Sci., 2022, 641, 119905 CrossRef CAS
.
- G. Polito, V. Robbiano, C. Cozzi, F. Cacialli and G. Barillaro, Sci. Rep., 2017, 7, 8351 CrossRef PubMed
.
- A. M. Darwish, A. Burkett, A. Blackwell, K. Taylor, S. Sarkisov, D. Patel, B. Koplitz and D. Hui, Composites, Part B, 2015, 68, 355–364 CrossRef CAS
.
- E. Caicedo-Casso, J. Sargent, R. M. Dorin, U. B. Wiesner, W. A. Phillip, B. W. Boudouris and K. A. Erk, J. Appl. Polym. Sci., 2019, 136, 47038 CrossRef
.
- K. Pandey, M. M. Dwivedi, S. S. Sanjay and N. Asthana, Ionics, 2017, 23, 113–120 CrossRef CAS
.
- Y. Lv, Y. Li, G. Zhang, Z. Peng, L. Ye, Y. Chen, T. Zhang, G. Xing and L. Chen, CCS Chem., 2021, 4, 1519–1525 CrossRef
.
- J. Chen, M. Guan, K. Li and S. Tang, ACS Appl. Mater. Interfaces, 2020, 12, 15138–15144 CrossRef CAS PubMed
.
- G. Yang, Z. Zhang, C. Yin, X. Shi and Y. Wang, J. Membr. Sci., 2022, 661, 120944 CrossRef CAS
.
- J. Wu, Y. Wang, Y. Wu, W. Xu, J. Wang, S. Li and Z. Xu, J. Membr. Sci., 2023, 687, 122091 CrossRef CAS
.
- M. Di, X. Sun, L. Hu, L. Gao, J. Liu, X. Yan, X. Wu, X. Jiang and G. He, Adv. Funct. Mater., 2022, 32, 2111594 CrossRef CAS
.
- S. Hao, J. Wen, S. Li, J. Wang and Z. Jia, J. Mater. Sci., 2020, 55, 14817–14828 CrossRef CAS
.
- B. Li, Z. Wang, Z. Gao, J. Suo, M. Xue, Y. Yan, V. Valtchev, S. Qiu and Q. Fang, Adv. Funct. Mater., 2023, 33, 2300219 CrossRef CAS
.
- W. Liu, Y. An, L. Wang, T. Hu, C. Li, Y. Xu, K. Wang, X. Sun, H. Zhang, X. Zhang and Y. Ma, J. Energy Chem., 2023, 80, 68–76 CrossRef CAS
.
- F. Wu, J. Li, Y. Su, J. Wang, W. Yang, N. Li, L. Chen, S. Chen, R. Chen and L. Bao, Nano Lett., 2016, 16, 5488–5494 CrossRef CAS PubMed
.
- D. D. Kulkarni, I. Choi, S. S. Singamaneni and V. V. Tsukruk, ACS Nano, 2010, 4, 4667–4676 CrossRef CAS PubMed
.
- X. Gong, G. Zhang, H. Dong, H. Wang, J. Nie and G. Ma, J. Membr. Sci., 2022, 657, 120667 CrossRef CAS
.
- K. Banjerdteerakul, H. Peng and K. Li, J. Membr. Sci., 2023, 681, 121780 CrossRef CAS
.
- S. Wang, Y. Yang, X. Liang, Y. Ren, H. Ma, Z. Zhu, J. Wang, S. Zeng, S. Song, X. Wang, Y. Han, G. He and Z. Jiang, Adv. Funct. Mater., 2023, 33, 2300386 CrossRef CAS
.
- J. Wu, S. Japip and T.-S. Chung, J. Mater. Chem. A, 2020, 8, 6196–6209 RSC
.
- F. Feng, J. Wu, C. Z. Liang, M. Weber, S. Zhang and T.-S. Chung, Chem. Eng. J., 2023, 470, 144073 CrossRef CAS
.
- S. T. Emmerling, R. Schuldt, S. Bette, L. Yao, R. E. Dinnebier, J. Kästner and B. V. Lotsch, J. Am. Chem. Soc., 2021, 143, 15711–15722 CrossRef CAS PubMed
.
- Z. Mu, Y. Zhu, B. Li, A. Dong, B. Wang and X. Feng, J. Am. Chem. Soc., 2022, 144, 5145–5154 CrossRef CAS PubMed
.
- H. Wang, L. Chen, H. Yang, M. Wang, L. Yang, H. Du, C. Cao, Y. Ren, Y. Wu, F. Pan and Z. Jiang, J. Mater. Chem. A, 2019, 7, 20317–20324 RSC
.
- H. Dou, M. Xu, B. Wang, Z. Zhang, G. Wen, Y. Zheng, D. Luo, L. Zhao, A. Yu, L. Zhang, Z. Jiang and Z. Chen, Chem. Soc. Rev., 2021, 50, 986–1029 RSC
.
- X. Shi, Z. Zhang, C. Yin, X. Zhang, J. Long, Z. Zhang and Y. Wang, Angew. Chem., Int. Ed., 2022, 61, e202207559 CrossRef CAS PubMed
.
- A. Xiao, X. Shi, Z. Zhang, C. Yin, S. Xiong and Y. Wang, J. Membr. Sci., 2021, 624, 119122 CrossRef CAS
.
- T. Chen, B. Li, W. Huang, C. Lin, G. Li, H. Ren, Y. Wu, S. Chen, W. Zhang and H. Ma, Sep. Purif. Technol., 2021, 256, 117787 CrossRef CAS
.
- L. Chen, C. Gong, X. Wang, F. Dai, M. Huang, X. Wu, C.-Z. Lu and Y. Peng, J. Am. Chem. Soc., 2021, 143, 10243–10249 CrossRef CAS PubMed
.
- V. A. Kuehl, J. Yin, P. H. H. Duong, B. Mastorovich, B. Newell, K.
D. Li-Oakey, B. A. Parkinson and J. O. Hoberg, J. Am. Chem. Soc., 2018, 140, 18200–18207 CrossRef CAS PubMed
.
- W. Xu, H. Zhuang, W. Chen, W. Liu and X. Pan, J. Appl. Polym. Sci., 2022, 139, e52767 CrossRef CAS
.
- M. Guan, Q. Liu, H. Zhang, Q. Li, J. Xu, M. Cai, W. Lin, W. Li and D. Yang, Desalination, 2023, 559, 116644 CrossRef CAS
.
- L. Xu, T. Yang, M. Li, J. Chang and J. Xu, J. Membr. Sci., 2020, 610, 118111 CrossRef CAS
.
- S. Han, W. You, S. Lv, C. Du, X. Zhang, E. Zhang, J. Zhu and Y. Zhang, Desalination, 2023, 548, 116300 CrossRef CAS
.
- X. Lin, Y. He, Y. Zhang, W. Yu and T. Lian, J. Membr. Sci., 2021, 638, 119725 CrossRef CAS
.
- Y. He, X. Lin, J. Chen and H. Zhan, J. Membr. Sci., 2021, 635, 119476 CrossRef CAS
.
- S. Li, P. Li, D. Cai, H. Shan, J. Zhao, Z. Wang, P. Qin and T. Tan, J. Membr. Sci., 2019, 579, 141–150 CrossRef CAS
.
- A. K. Mohammed, A. A. Al Khoori, M. A. Addicoat, S. Varghese, I. Othman, M. A. Jaoude, K. Polychronopoulou, M. Baias, M. A. Haija and D. Shetty, Angew. Chem., Int. Ed., 2022, 61, e202200905 CrossRef CAS PubMed
.
- L. Chen, J. Du, W. Zhou, H. Shen, L. Tan, C. Zhou and L. Dong, Chem. – Asian J., 2020, 15, 3421–3427 CrossRef CAS PubMed
.
- Y. Yang, J. Dong, R. Wang, Z. Lin and Z. Cai, J. Hazard. Mater., 2023, 459, 132149 CrossRef CAS PubMed
.
- N. Basel, Q. Liu, L. Fan, Q. Wang, N. Xu, Y. Wan, Q. Dong, Z. Huang and T. Guo, Sep. Purif. Technol., 2022, 303, 122243 CrossRef CAS
.
- M. Chen, K. Yang, J. Wang, H. Sun, X.-H. Xia and C. Wang, Adv. Funct. Mater., 2023, 33, 2302427 CrossRef CAS
.
- H. Fan, M. Peng, I. Strauss, A. Mundstock, H. Meng and J. Caro, J. Am. Chem. Soc., 2020, 142, 6872–6877 CrossRef CAS PubMed
.
- H. Fan, H. Wang, M. Peng, H. Meng, A. Mundstock, A. Knebel and J. Caro, ACS Nano, 2023, 17, 7584–7594 CrossRef CAS PubMed
.
- J. Wu and T. S. Chung, Small Methods, 2022, 6, 2101288 CrossRef CAS PubMed
.
- J. Fu, S. Das, G. Xing, T. Ben, V. Valtchev and S. Qiu, J. Am. Chem. Soc., 2016, 138, 7673–7680 CrossRef CAS PubMed
.
- S. Das, T. Ben, S. Qiu and V. Valtchev, ACS Appl. Mater. Interfaces, 2020, 12, 52899–52907 CrossRef CAS PubMed
.
- P. Wang, Y. Peng, C. Zhu, R. Yao, H. Song, L. Kun and W. Yang, Angew. Chem., Int.
Ed., 2021, 60, 19047–19052 CrossRef CAS PubMed
.
- Y. Zhang, L. Ma, Y. Lv and T. Tan, Chem. Eng. J., 2022, 430, 133001 CrossRef CAS
.
- J. Wu, F. Hillman, C.-Z. Liang, Y. Jia and S. Zhang, J. Mater. Chem. A, 2023, 11, 17452–17478 RSC
.
- Y. Ying, S. B. Peh, H. Yang, Z. Yang and D. Zhao, Adv. Mater., 2022, 34, 2104946 CrossRef CAS PubMed
.
- Y. Liu, H. Wu, R. Li, J. Wang, Y. Kong, Z. Guo, H. Jiang, Y. Ren, Y. Pu, X. Liang, F. Pan, Y. Cao, S. Song, G. He and Z. Jiang, Adv. Mater., 2022, 34, 2201423 CrossRef CAS PubMed
.
- H. Fan, M. Peng, I. Strauss, A. Mundstock, H. Meng and J. Caro, Nat. Commun., 2021, 12, 38 CrossRef CAS PubMed
.
- H. Yun, M. Kang, D. W. Kang, H. Kim, J. H. Choe, S. Y. Kim and C. S. Hong, Small, 2023, 19, 2303640 CrossRef CAS PubMed
.
- Z. Guo, H. Wu, Y. Chen, S. Zhu, H. Jiang, S. Song, Y. Ren, Y. Wang, X. Liang, G. He, Y. Li and Z. Jiang, Angew. Chem., Int. Ed., 2022, 61, e202210466 CrossRef CAS PubMed
.
- D. Liu, K. Li, M. Li, Z. Wang, M. Shan and Y. Zhang, ACS Appl. Mater. Interfaces, 2021, 13, 37775–37784 CrossRef CAS PubMed
.
- D. L. Zhao, F. Feng, L. Shen, Z. Huang, Q. Zhao, H. Lin and T.-S. Chung, Chem. Eng. J., 2023, 454, 140447 CrossRef CAS
.
- Y.-Y. Su, X. Yan, Y. Chen, X.-J. Guo, X.-F. Chen and W.-Z. Lang, J. Membr. Sci., 2021, 618, 118706 CrossRef CAS
.
- J. Shen, J. Yuan, B. Shi, X. You, R. Ding, T. Zhang, Y. Zhang, Y. Deng, J. Guan, M. Long, Y. Zheng, R. Zhang, H. Wu and Z. Jiang, J. Mater. Chem. A, 2021, 9, 23178–23187 RSC
.
- G. Zhang, G. Chen, M. Dong, J. Nie and G. Ma, ACS Appl. Mater. Interfaces, 2023, 15, 32903–32915 CrossRef CAS PubMed
.
- K. Banjerdteerakul, H. Peng and K. Li, J. Membr. Sci., 2023, 683, 121852 CrossRef CAS
.
- Y. Zhang, J. Guo, G. Han, Y. Bai, Q. Ge, J. Ma, C. H. Lau and L. Shao, Sci. Adv., 2021, 7, eabe8706 CrossRef CAS PubMed
.
- L.-C. Lin, J. Choi and J. C. Grossman, Chem. Commun., 2015, 51, 14921–14924 RSC
.
- R. Wang, M. Wei and Y. Wang, J. Membr. Sci., 2020, 604, 118090 CrossRef CAS
.
- F.-X. Kong, L. Yue, Z. Yang, G. Sun and J.-F. Chen, ACS Appl. Mater. Interfaces, 2021, 13, 21379–21389 CrossRef CAS PubMed
.
- Y. Yin, S. Liu, J. Zhou, Y. Peng, E. Wang, L. Han and B. Su, J. Membr. Sci., 2023, 686, 122000 CrossRef CAS
.
- C. Cao, H. Wang, M. Wang, Y. Liu, Z. Zhang, S. Liang, W. Yuhan, F. Pan and Z. Jiang, J. Membr. Sci., 2021, 630, 119319 CrossRef CAS
.
- K. Banjerdteerakul, H. Peng and K. Li, J. Membr. Sci., 2023, 678, 121679 CrossRef CAS
.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |