Open Access Article
Open Access ArticleLemniscular carbon nanohoops with contiguous conjugation from planar chiral [2.2]paracyclophane: influence of the regioselective synthesis on topological chirality†
Jing
He
a,
Mo-Han
Yu
b,
Zhe
Lian
a,
Yan-Qing
Fan
a,
Sheng-Zhu
Guo
a,
Xiao-Nan
Li
a,
Ying
Wang
 a,
Wen-Guang
Wang
a,
Wen-Guang
Wang
 a,
Zhi-Yun
Cheng
c and
Hua
Jiang
a,
Zhi-Yun
Cheng
c and
Hua
Jiang
 *a
*a
aCollege of Chemistry, Beijing Normal University, Beijing 100875, P. R. China. E-mail: jiangh@bnu.edu.cn
bCollege of Chemistry and Chemical Engineering, Liaoning Normal University, Dalian 116029, P. R. China
cSchool of Mathematical Sciences, Beijing Normal University, Beijing 100875, P. R. China
First published on 28th March 2023
Abstract
We report herein the regioselective synthesis of all-carbon lemniscular nanohoops bis-po-CC and bis-pm-TC by the rational control of ring closures at the different positions of planar chiral tetrasubstituted [2.2]paracyclophane. Topological analyses reveal that bis-pm-TC is topologically chiral while bis-po-CC is topologically achiral. X-ray crystal analysis demonstrates that bis-pm-TC adopts a lemniscular conformation with a contiguous conjugation. CD and CPL measurements further reveal that the chiroptical properties of bis-pm-TC are obviously different from those of bis-po-CC due to their different topological chiralities.
Introduction
Molecular architectures with fascinating topological chirality have attracted considerable attention because of their aesthetic appeal, synthetic challenge,1 and potential applications in electronic devices2 and molecular machines.3 Compared to Euclidean chiral molecules having the classical stereogenic units such as points, axes, helicenes and planes, topologically chiral molecules require that their mirror image presentations are topologically distinct. Therefore, it is necessary to have nonplanar molecular graphs which cannot be converted into their mirror images by continuous deformation in 3D space without cutting.1,4 Over the past few decades, the synthesis of topologically chiral molecules has long been dominated by mechanically interlocked molecules (MIMs),1,5 which were conventionally synthesized by means of several reversible interactions such as active-metal templates, metal-ion templates, electrostatic interactions, π–π stacking or hydrogen bonding,6 thus resulting in non-conjugated MIMs. In sharp contrast, synthesis of conjugated all-carbon architectures with topological chirality remains largely unexplored due to their synthetic challenge and structural complexity. Limited examples of conjugated carbon nanohoops with Möbius topology have been developed so far.7 Therefore, there is a huge demand for developing novel strategies for accessing topologically chiral all-carbon macrocycles with aesthetic structural features, and eventually distinctive applications as functional nanocarbon materials.The rapid development of cycloparaphenylenes (CPPs)8 provides an excellent platform for developing topological conjugated nanocarbons. To date, a few elegant examples of CPP-based “figure-eight” or lemniscular conjugated architectures have been developed by introducing 3D covalent building blocks such as pentiptycene,9a bicarbazole,9b,c spirobifluorene,9d biphenyl,9e and tetraphenylbenzene9f units into the CPP backbone. Those obtained conjugated macrocycles are Euclidean chiral, but no finding on topological chirality has been disclosed.9 Remarkably, Itami et al. recently reported the first synthesis of a topologically chiral all-benzene trefoil knot from CPPs by using a 3D spirosilane-assisted traceless synthetic method.10
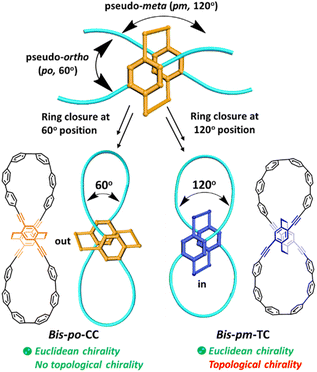
Inspired by significant advances on CPP derived architectures, we envisioned a straightforward approach by introducing a chiral 3D building block into CPP backbones to design topologically chiral lemniscular carbon nanohoops. To this end, 4,7,12,15-tetrasubstituted [2.2]paracyclophane (PCP) is considered as a promising chiral 3D candidate because its diverse substituent positions and unique planar chirality11 allow us to fulfill regioselective synthesis and to control the topological chirality of targeted nanohoops. However, none of the PCP-based macrocycles reported so far dealt with topological chirality,12 including our previously reported semi-macrocycles PCP-[n]CPP,12e which are also topologically trivial (vide infra, Fig. S2†). Specifically, planar chiral 4,7,12,15-tetrasubstituted [2.2]PCP demonstrates a 60° orientation between pseudo-ortho(po) substituents and a 120° orientation between pseudo-meta(pm) substituents (Scheme 1). The obviously different orientations thus provide an appealing option to synthesize lemniscular nanohoops bis-po-CC and bis-pm-TC by regioselective ring closures (Scheme 1 and 2), leading to formation of a lemniscular framework with a differently orienting [2.2]PCP core. In detail, the ethylene bridges of the [2.2]PCP core in bis-po-CC are located out of the lemniscular framework, while those in bis-pm-TC are encircled by the lemniscular framework, reminiscent of interlocked conformations in MIMs (Scheme 1). Herein, we report the regioselective synthesis of isomers bis-po-CC and bis-pm-TC by the rational control of ring closures (Scheme 1 and 2) and our efforts to analyse the influence of regioselective synthesis on their topological chirality via molecular graphs obtained by Kauffman's approach (Fig. 2, vide infra). We found that the regioselective synthesis exerts a significant influence on the topological chirality of bis-pm-TC and bis-po-CC.
Results and discussion
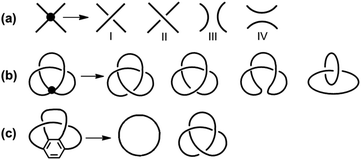
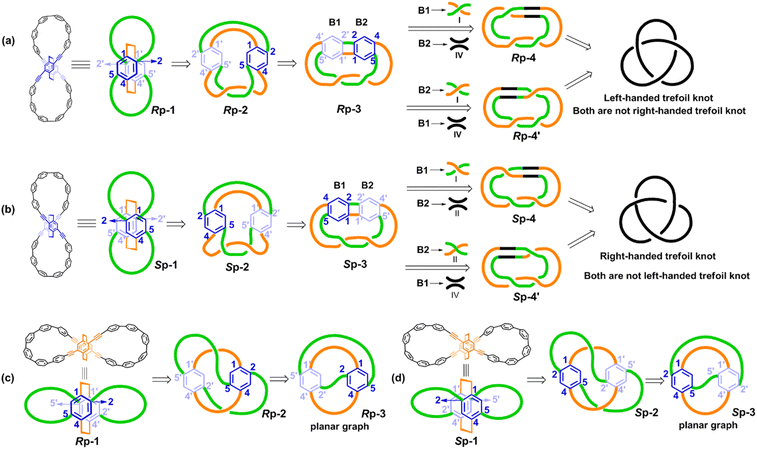
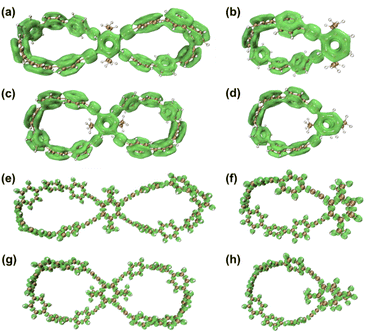
Unlike the most classic topologically chiral molecules such as trefoil knots, whose topology and molecular graph are intuitional and canonical, those of bis-po-CC and bis-pm-TC are not, and thus their topological chiralities need to be detected by means of topological analysis. In this context, we utilized the topological approach proposed by L. Kauffman,13 and further developed by E. Flapan1b to analyse the topological chirality of bis-po-CC and bis-pm-TC. Kauffman defined an invariant which could be used for detecting the topological chirality of rigid vertex embedded graphs. For an embedded four-valent rigid vertex graph G, C(G) is the collection of knots and links associated with G by replacing all of the vertex disks and the four strands emanating from them with each one of the four pictures on the right hand side of Fig. 1a. Kauffman proved that the set C(G) is a topological invariant of any embedded rigid vertex graph G.13 Accordingly, if there is a topologically chiral element of C(G) that cannot be deformed to the mirror image of any other element of C(G), then G is topologically chiral. A rigid vertex embedded graph (G1) was taken as an example shown in Fig. 1b, whose C(G1) was defined to be the collection of knots and links associated with G1 by replacing the rigid vertex with each one of the four pictures on the right side of Fig. 1a. The elements of C(G1) as shown in Fig. 1b contain a trefoil knot but without its mirror image. Therefore, the rigid vertex embedded graph (G1) is topologically chiral. Lately, Flapan developed Kauffman's topological approach by treating a four substituted benzene ring as the vertex disk and created an imaginary molecule in the form of Fig. 1c. Accordingly, the benzene ring would act as a rigid vertex disk, and as such the graph would be topologically chiral because it contains a trefoil knot but not its mirror image.1bBased on the aforementioned topological approach, in which the tetrasubstituted benzene ring acts as a four-valent rigid vertex disk (Fig. 1b), we first address how to detect the topological chirality of bis-po-CC and bis-pm-TC. So the tetrasubstituted benzene ring of bis-po-CC and bis-pm-TC can be replaced by each of the four pictures (I/II/III/IV) in the four-valent rigid vertex disk, respectively, as shown in Fig. 1b. We take the topological transformation of RP-bis-pm-TC into a left-handed trefoil knot graph as an example to illustrate this topological approach as shown in Fig. 2a. The carbon nanohoop RP-bis-pm-TC is reducible to graph Rp-1. The top benzene can be moved to the right to generate graph Rp-2 containing two alternating crossings. In graph Rp-2, the left benzene ring is rotated 90° clockwise, while the right one is rotated anticlockwise to generate graph Rp-3. Subsequently, by replacing benzene rings B1 and B2 with the pictures I and IV in Fig. 1a, respectively, the graphs Rp-4 and Rp-4’ are obtained easily and topologically equivalent to a left-handed trefoil knot. The remaining graphs of bis-pm-TC can be obtained by a similar topological approach, and are depicted in Fig. S3.† All topologically trivial and nontrivial graphs of RP-bis-pm-TC are summarized in Table 1. Pleasingly, no right-handed trefoil knot is found in all graphs for RP-bis-pm-TC, indicating that RP-bis-pm-TC is topologically chiral. Similarly, SP-bis-pm-TC contains only a right-handed trefoil knot graph but not its mirror image and thus is topologically chiral (Fig. 2b, S4 and Table S2†). Moreover, a graph of Solomon links is also obtained for bis-pm-TC, further confirming that bis-pm-TC has topological chirality (Fig. S3†). Accordingly, the molecular graphs of the precursor bis-pm-11 (Scheme 2) are the same as those of bis-pm-TC (figures not shown), and thus bis-pm-11 is also topologically chiral. However, the topologic transformations of RP-/SP-bis-po-CC only generate a planar graph (Fig. 2c and d), and thus no topologically chiral graph is found for bis-po-CC (Fig. S5 and Table S3†), suggesting that bis-po-CC is topologically achiral. The above topological analyses clearly demonstrate that the orientations of the 4,7,12,15-tetrasubstituted [2.2]PCP core resulting from the regioselective synthesis in the lemniscular conformation play a vital role in controlling the topological chirality of conjugated carbon nanohoops.
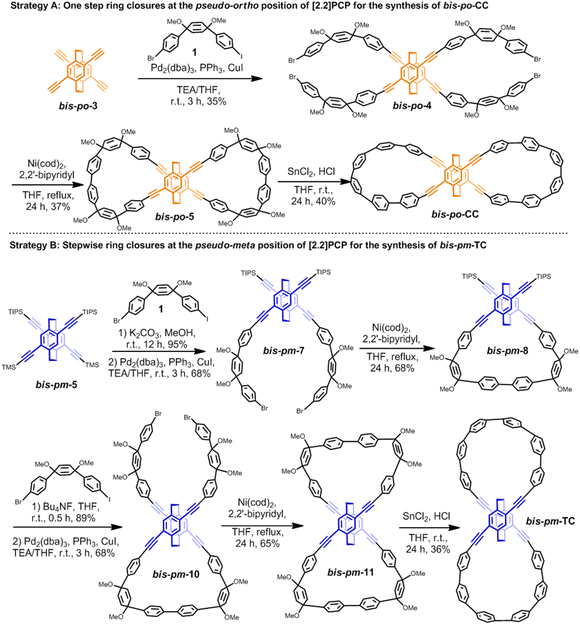
With the results from topological analysis in hand, we next set about synthesizing bis-po-CC and bis-pm-TC by regioselective synthesis (Scheme 2). For the synthesis of bis-po-CC (Scheme 2, Strategy A), we commenced with the Sonogashira–Hagihara coupling reaction with compounds bis-po-314 and 112e to afford intermediate bis-po-4 in 35% yield, which then underwent Yamamoto coupling to give the macrocyclic precursor bis-po-5 (in 37% yield) as the dominant product in which the ring closures occurred at the pseudo-ortho position of [2.2]PCP. Unsurprisingly, no product with the ring closures at the pseudo-meta position was detected and isolated due to the larger distance for ring closure at this position. The final reductive aromatization was carried out using a freshly prepared H2SnCl4 solution, providing bis-po-CC as a yellow solid in 40% yield.
In order to synthesize bis-pm-TC, we adopted step-wise ring closures at the pseudo-meta positions of 4,15-bis[(trimethylsilyl)ethynyl]-7,12-bis[(triisopropylsilyl) ethynyl] [2.2]PCP (Scheme 2: Strategy B) obtained by selective deprotection of TMS/TIPS-ethynyl groups. First, TMS groups in bis-pm-5 were selectively removed by K2CO3/MeOH to obtain4,15-bis(ethynyl)-7,12-bis(TIPS-ethynyl) [2.2]PCP and then underwent Sonogashira–Hagihara coupling with 1 to generate bis-pm-7, which was further converted to semi-macrocyclic intermediate bis-pm-8 through Yamamoto coupling. The precursor bis-pm-11 was obtained by similar synthetic procedures after the deprotection of TIPS groups in bis-pm-8. The final reductive aromatization in a freshly prepared H2SnCl4 solution produced the desired bis-pm-TC as a yellow solid.
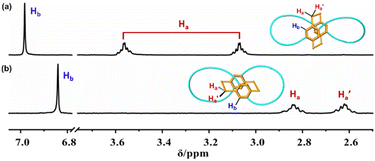
1H-NMR spectra of bis-po-CC and bis-pm-TC reveal a sharp difference in chemical shifts of all protons (Fig. 3 and S6†). Particularly, the 1H NMR spectrum of bis-po-CC shows a pair of multiplets at 3.59 and 3.04 ppm assignable to the protons of ethylene bridges and a singlet at 6.98 ppm assignable to the protons of benzene rings in the [2.2]PCP core. In contrast, the 1H-NMR spectrum of bis-pm-TC displays a pair of multiplets at 2.87 and 2.58 ppm and a singlet at 6.84 ppm belonging to the protons of ethylene bridges and benzene rings, respectively. The data demonstrate that the protons of ethylene bridges in bis-pm-TC display significant upfield chemical shifts with Δδ up to 0.72 ppm in comparison with those in bis-po-CC. The significant upfield shifts of the protons of ethylene bridges can be interpreted as the shielding effects from lemniscular nanohoops (Scheme 1 and 2).
The X-ray crystallographic analysis of single crystals of bis-pm-11 and bis-pm-TC, obtained by the slow vapor diffusion of hexane into a CHCl3 solution at room temperature, gave unequivocal confirmation of their topological structures. The structural analysis revealed that bis-pm-11 shows a bow-shaped conformation (Fig. 4a). Its unit cell is triclinic with a space group of P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with two opposite enantiomers in a crystal cell (Fig. S11†). The enantiomers of bis-pm-11 adopt an interlaced network in the solid state (Fig. 4b). The crystal analysis revealed that bis-pm-TC adopts a twisted lemniscular structure (Fig. 4c). The unit cell is monoclinic with a space group of P21/n. Examination of the solid-state packing of bis-pm-TC reveals the ordered supramolecular alignment of long-channels (Fig. 4d). Many attempts to grow suitable crystals of bis-po-CC failed.
with two opposite enantiomers in a crystal cell (Fig. S11†). The enantiomers of bis-pm-11 adopt an interlaced network in the solid state (Fig. 4b). The crystal analysis revealed that bis-pm-TC adopts a twisted lemniscular structure (Fig. 4c). The unit cell is monoclinic with a space group of P21/n. Examination of the solid-state packing of bis-pm-TC reveals the ordered supramolecular alignment of long-channels (Fig. 4d). Many attempts to grow suitable crystals of bis-po-CC failed.
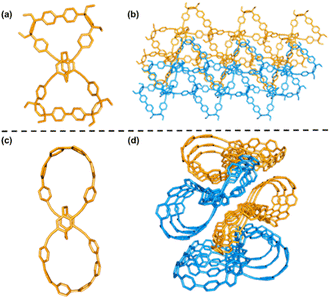 | ||
| Fig. 4 X-ray crystal structures of the bow-shaped bis-pm-11 (a) and its crystal packing (b). X-ray crystal structures of the figure-eight shaped bis-pm-TC (c) and its crystal packing (d). | ||
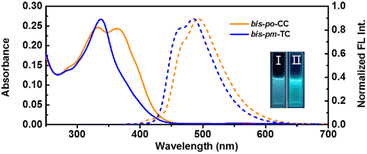
The photophysical properties of bis-po-CC and bis-pm-TC were investigated in dichloromethane solutions (Fig. 5). The absorption spectrum of bis-po-CC exhibits two major absorption peaks at 334 nm (ε = 2.5 × 105 M−1 cm−1) and 361 nm (ε = 2.4 × 105 M−1 cm−1), which are ascribed to the electronic transitions of HOMO−2 → LUMO+2 (λDFT = 328 nm) and HOMO−2 → LUMO (λDFT = 367 nm), respectively, according to time-dependent density functional theory (TD-DFT) calculations (Fig. S18 and Table S9†). In contrast, bis-pm-TC shows only one intense absorption band at 338 nm (ε = 2.7 × 105 M−1 cm−1) correlating with the transitions of HOMO → LUMO and HOMO−2 → LUMO+1 (λDFT = 341 nm) (Fig. S19 and Table S11†).
The fluorescence emission spectrum of bis-po-CC exhibits one emission band with a maximum peak at 495 nm under excitation at 330 nm (Fig. 5), while the emission spectrum of bis-pm-TC shows a slight blueshift feature with a maximum peak at 484 nm. It is worth noting that these emission peaks obviously shifted towards lower energy in comparison with their semi-macrocycle pm-PCP-[6]CPP (λem = 472 nm),12e indicative of a contiguous conjugation in the lemniscular conformations. The fluorescence quantum yields of bis-po-CC and bis-pm-TC were determined to be 33% and 51%, respectively. In addition, the fluorescence lifetimes (τ) of bis-po-CC and bis-pm-TC were calculated to be 2.4 and 3.2 ns, respectively, fitting with a single exponential relationship (Fig. S15†).
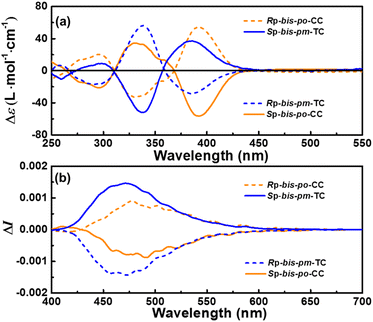
The enantiomers of bis-po-CC and bis-pm-TC were separated by chiral HPLC (Fig. S13 and S14†), respectively, and their CD spectra are depicted in Fig. 6a. The absolute configurations of the enantiomers were assigned according to TD-DFT calculations (Fig. S20†). The CD spectra of enantiomers bis-po-CC and bis-pm-TC feature bands with variable intensities between 250 and 450 nm with a maximum at 393 nm (|Δε| = 54 M−1 cm−1) for bis-po-CC and at 337 nm (|Δε| = 57 M−1 cm−1) for bis-pm-TC (Fig. 6a). The CD spectrum of RP-bis-po-CC shows the positive of negative sign for any given wavelength. Interestingly, the CD spectrum of RP-bis-pm-TC displays bands with an inverted sign, suggesting a chirality opposite to that of RP-bis-po-CC even though they contain the same planar chiral RP-[2.2] PCP core. The inverted sign can be interpreted as the different orientations of the [2.2] PCP core in the lemniscular carbon nanohoops and as the topological chirality of RP-bis-pm-TC. The CD spectra of SP- enantiomers exhibit the mirror images of those of RP-ones for both carbon nanohoops. The dissymmetry factors |gabs| = |Δε/ε| were calculated to be 3.1 × 10−3 (390 nm) for bis-po-CC and 2.9 × 10−3 (387 nm) for bis-pm-TC. The circularly polarized luminescence (CPL) spectra reveal that RP-bis-po-CC shows one positive CPL signal in the range of 400–700 nm, whereas RP-bis-pm-TC displays one negative signal in the same region (Fig. 6b), consistent with the observations from the ECD results. Similarly, the CPL spectra of SP- enantiomers exhibited the mirror images of those of RP-ones. The CPL dissymmetry factor (|glum|) was determined as 1.8 × 10−3 at 491 nm for bis-po-CC and 3.2 × 10−3 at 472 nm for bis-pm-TC, respectively. The larger |glum| value for bis-pm-TC might be ascribed to the more rigid structure of bis-pm-TC in the excited state.12e Moreover, the |glum| values are comparable with those of most of the CPL-active small organic molecules, which are typically in the range of 10−2 to 10−4 (Fig. S16 and Table S6†).15 However, it is very challenging to differentiate topological chirality from chemical one in bis-pm-TC by experimental methods because the nanohoop contains a classic stereogenic motif. Such challenges also exist in other reported topologically chiral MIMs and organic trefoil knots.16
The localized orbital locator (LOL)17 provides an intuitive depiction of isolated electron densities of π-electrons. The LOL isosurfaces of semi-macrocycles po-PCP-[6]CPP and pm-PCP-[6]CPP were calculated for comparison with lemniscular nanohoops bis-po-CC and bis-pm-TC. In the case of bis-po-CC and bis-pm-TC, contiguously delocalized π-electron density is accumulated in all benzene rings at LOL-π isovalue = 0.30 level (Fig. 7). At the same isovalue level, for the semi-macrocycle, the π-electrons also delocalize over almost all the benzene rings including the [2.2] PCP core (Fig. S22†). However, LOL-π investigations revealed a contiguously circular conjugation for both bis-po-CC and bis-pm-TC with a lemniscular framework but an acyclic conjugation for semi-macrocycles po/pm-PCP-[6]CPP, indicative of a larger extent of delocalization for the former. The same arguments also hold for LOL-σ plots at isovalue = 0.60 for all nanohoops (Fig. 7 and S23†). The LOL investigations are in good agreement with the results of the red-shift in fluorescence emission.
Data availability
Data available in the ESI.†Conclusions
In conclusion, we have reported the regioselective synthesis of lemniscular carbon nanohoops bis-po-CC and bis-pm-TC by the judicious control of the ring closures at the different positions of the 3D planar chiral tetrasubstituted [2.2]PCP. The topological analysis demonstrated that bis-pm-TC is topologically chiral but bis-po-CC not. Interestingly, CD and CPL experiments revealed that chiral isomers RP(SP)-bis-po-CC and RP(SP)-bis-pm-TC exhibited opposite chiral signals even though they contain the same planar chiral RP(SP)-[2.2] PCP cores in the lemniscular nanohoops. This unique phenomenon originates from the different orientations of the imbedded [2.2] PCP core in the lemniscular nanohoops and their different topological chiralities. This work provides a new guideline for design and synthesis of topologically chiral all-carbon molecules.Author contributions
J. H. synthesized and characterized all materials. M.-H. Y. performed DFT calculations and simulations of photophysical spectra. Z. L. and Y.-Q. F. collected X-ray data. S.-Z. G. and X.-N. L synthesized partial material. Y. W. performed DFT calculations of LOL isosurfaces. W.-G. W. helped to analyze some of the experimental data. Z.-Y. C. gave guidance about the topological analyses. H. J. supervised this project and revised the manuscript. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Beijing Natural Science Foundation (2212008) and the National Natural Science Foundation of China (21971020 and 22271019).References
- (a) Molecular Catenanes, Rotaxanes and Knots, ed. J.-P. Sauvage and C. Dietrich-Buchecker, Wiley-VCH, Weinheim, Germany, 1999 Search PubMed; (b) When Topology Meets Chemistry: A Topological Look at Molecular Chirality, ed. E. Flapan, Cambridge University Press, Cambridge, England, 2000 Search PubMed; (c) F. Vögtle and O. Lukin, Angew. Chem., Int. Ed., 2005, 44, 1456 CrossRef PubMed; (d) R. S. Forgan, J.-P. Sauvage and J. F. Stoddart, Chem. Rev., 2011, 111, 5434 CrossRef CAS PubMed.
- A. Coskun, J. M. Spruell, G. Barin, W. R. Dichtel, A. H. Flood, Y. Y. Botros and J. F. Stoddart, Chem. Soc. Rev., 2012, 41, 4827 RSC.
- (a) S. Erbas-Cakmak, D. A. Leigh, C. T. McTernan and A. L. Nussbaumer, Chem. Rev., 2015, 115, 10081 CrossRef CAS PubMed; (b) J.-P. Sauvage, Angew. Chem., Int. Ed., 2017, 56, 11080 CrossRef CAS.
- (a) H. L. Frisch and E. Wasserman, J. Am. Chem. Soc., 1961, 83, 3789 CrossRef CAS; (b) D. M. Walba, Tetrahedron, 1985, 41, 3161 CrossRef CAS; (c) D. K. Mitchell and J.-P. Sauvage, Angew. Chem., Int. Ed., 1988, 27, 930 CrossRef; (d) J. C. Chambron, C. O. Dietrich-Buchecker and J.-P. Sauvage, Top. Curr. Chem., 1993, 165, 132 CrossRef; (e) C. Liang and K. Mislow, J. Am. Chem. Soc., 1994, 116, 3588 CrossRef CAS; (f) C. Dietrich-Buchecker, G. Rapenne, J.-P. Sauvage, A. De Cian and J. Fischer, Chem. – Eur. J., 1999, 5, 1432 CrossRef CAS.
- For selected reviews, see: (a) F. M. Raymo and J. F. Stoddart, Chem. Rev., 1999, 99, 1643 CrossRef CAS PubMed; (b) J. F. Stoddart, Chem. Soc. Rev., 2009, 38, 1802 RSC; (c) G. Gil-Ramírez, D. A. Leigh and A. J. Stephens, Angew. Chem., Int. Ed., 2015, 54, 6110 CrossRef PubMed; (d) S. D. P. Fielden, D. A. Leigh and S. L. Woltering, Angew. Chem., Int. Ed., 2017, 56, 11166 CrossRef CAS; (e) E. M. G. Jamieson, F. Modicom and S. M. Goldup, Chem. Soc. Rev., 2018, 47, 5266 RSC; (f) S. Mena-Hernando and E. M. Pérez, Chem. Soc. Rev., 2019, 48, 5016 RSC.
- (a) J. C. Chambron and J.-P. Sauvage, New J. Chem., 2013, 37, 49 RSC; (b) J.-F. Ayme, J. E. Beves, C. J. Campbell and D. A. Leigh, Chem. Soc. Rev., 2013, 42, 1700 RSC; (c) The Nature of the Mechanical Bond: From Molecules to Machines, ed. C. J. Bruns and J. F. Stoddart, John Wiley & Sons, Inc., Hoboken, 2016 Search PubMed; (d) H.-N. Zhang, W.-X. Gao, Y.-J. Lin and G.-X. Jin, J. Am. Chem. Soc., 2019, 141, 16057 CrossRef CAS PubMed; (e) M. Denis, J. E. M. Lewis, F. Modicom and S. M. Goldup, Chem, 2019, 5, 1512 CrossRef CAS PubMed; (f) Z. Cui, Y. Lu, X. Gao, H.-J. Feng and G.-X. Jin, J. Am. Chem. Soc., 2020, 142, 13667 CrossRef CAS PubMed; (g) J. R. J. Maynard and S. M. Goldup, Chem, 2020, 6, 1914 CrossRef CAS; (h) W.-X. Gao, H.-J. Feng, B.-B. Guo and G.-X. Jin, Chem. Rev., 2020, 120, 6288 CrossRef CAS PubMed; (i) Q.-H. Guo, Y. Jiao, Y. Feng and J.-F. Stoddart, CCS Chem., 2021, 3, 1542 CrossRef CAS; (j) A. Rodriguez-Rubio, A. Savoini, F. Modicom, P. Butler and S. M. Goldup, J. Am. Chem. Soc., 2022, 144, 11927 CrossRef CAS; (k) Y. Wang, J. Gong, X. Wang, W.-J. Li, X.-Q. Wang, X. He, W. Wang and H.-B. Yang, Angew. Chem., Int. Ed., 2022, 61, e202210542 CAS.
- (a) G. R. Schaller, F. Topić, K. Rissanen, Y. Okamoto, J. Shen and R. Herges, Nat. Chem., 2014, 6, 608 CrossRef CAS PubMed; (b) G. Naulet, L. Sturm, A. Robert, P. Dechambenoit, F. Röhricht, R. Herges, H. Bock and F. Durola, Chem. Sci., 2018, 9, 8930 RSC; (c) S. Nishigaki, Y. Shibata, A. Nakajima, H. Okajima, Y. Masumoto, T. Osawa, A. Muranaka, H. Sugiyama, A. Horikawa, H. Uekusa, H. Koshino, M. Uchiyama, A. Sakamoto and K. Tanaka, J. Am. Chem. Soc., 2019, 141, 14955 CrossRef CAS PubMed; (d) X. Jiang, J. D. Laffoon, D. Chen, S. Pérez-Estrada, A. S. Danis, J. Rodríguez-López, M. A. Garcia-Garibay, J. Zhu and J. S. Moore, J. Am. Chem. Soc., 2020, 142, 6493 CrossRef CAS PubMed; (e) Y. Segawa, T. Watanabe, K. Yamanoue, M. Kuwayama, K. Watanabe, J. Pirillo, Y. Hijikata and K. Itami, Nat. Synth., 2022, 1, 535 CrossRef.
- For selected reviews, see: (a) H. Omachi, Y. Segawa and K. Itami, Acc. Chem. Res., 2012, 45, 1378 CrossRef CAS PubMed; (b) S. Yamago, E. Kayahara and T. Iwamoto, Chem. Rec., 2014, 14, 84 CrossRef CAS PubMed; (c) M. R. Golder and R. Jasti, Acc. Chem. Res., 2015, 48, 557 CrossRef CAS PubMed; (d) E. R. Darzi and R. Jasti, Chem. Soc. Rev., 2015, 44, 6401 RSC; (e) S. E. Lewis, Chem. Soc. Rev., 2015, 44, 2221 RSC; (f) Y. Segawa, A. Yagi, K. Matsui and K. Itami, Angew. Chem., Int. Ed., 2016, 55, 5136 CrossRef CAS PubMed; (g) D. Wu, W. Cheng, X. Ban and J. Xia, Asian J. Org. Chem., 2018, 7, 2161 CrossRef CAS; (h) M. Hermann, D. Wassy and B. Esser, Angew. Chem., Int. Ed., 2021, 60, 15743 CrossRef CAS PubMed.
- (a) W. Xu, X.-D. Yang, X.-B. Fan, X. Wang, C.-H. Tung, L.-Z. Wu and H. Cong, Angew. Chem., Int. Ed., 2019, 58, 3943 CrossRef CAS; (b) K. Senthilkumar, M. Kondratowicz, T. Lis, P. J. Chmielewski, J. Cybinska, J. L. Zafra, J. Casado, T. Vives, J. Crassous, L. Favereau and M. Stępień, J. Am. Chem. Soc., 2019, 141, 7421 CrossRef CAS PubMed; (c) L. Palomo, L. Favereau, K. Senthilkumar, M. Stępień, J. Casado and F. J. Ramírez, Angew. Chem., Int. Ed., 2022, 61, e202206976 CrossRef CAS PubMed; (d) T. A. Schaub, E. A. Prantl, J. Kohn, M. Bursch, C. R. Marshall, E. J. Leonhardt, T. C. Lovell, L. N. Zakharov, C. K. Brozek, S. R. Waldvogel, S. Grimme and R. Jasti, J. Am. Chem. Soc., 2020, 142, 8763 CrossRef PubMed; (e) L.-H. Wang, N. Hayase, H. Sugiyama, J. Nogami, H. Uekusa and K. Tanaka, Angew. Chem., Int. Ed., 2020, 59, 17951 CrossRef CAS PubMed; (f) X. Zhang, H. Liu, G. Zhuang, S. Yang and P. Du, Nat. Commun., 2022, 13, 3543 CrossRef CAS PubMed.
- Y. Segawa, M. Kuwayama, Y. Hijikata, M. Fushimi, T. Nishihara, J. Pirillo, J. Shirasaki, N. Kubota and K. Itami, Science, 2019, 365, 272 CrossRef CAS PubMed.
- (a) Z. Hassan, E. Spuling, D. M. Knoll and S. Bräse, Angew. Chem., Int. Ed., 2020, 59, 2156 CrossRef CAS PubMed; (b) J.-M. Teng, D.-W. Zhang and C.-F. Chen, ChemPhotoChem, 2022, 6, e202100228 CrossRef CAS.
- Examples see: (a) Y. Morisaki, M. Gon, T. Sasamori, N. Tokitoh and Y. Chujo, J. Am. Chem. Soc., 2014, 136, 3350 CrossRef CAS; (b) K. J. Weiland, T. Brandl, K. Atz, A. Prescimone, D. Haussinger, T. Solomek and M. Mayor, J. Am. Chem. Soc., 2019, 141, 2104 CrossRef CAS PubMed; (c) Y. Wu, G. Zhuang, S. Cui, Y. Zhou, J. Wang, Q. Huang and P. Du, Chem. Commun., 2019, 55, 14617 RSC; (d) K. Tanaka, R. Inoue and Y. Morisaki, Chem. – Asian J., 2022, 17, e202101267 CrossRef CAS PubMed; (e) J. He, M. Yu, M. Pang, Y. Fan, Z. Lian, Y. Wang, W. Wang, Y. Liu and H. Jiang, Chem. – Eur. J., 2022, 28, e202103832 CAS; (f) E. Sidler, P. Zwick, C. Kress, K. Reznikova, O. Fuhr, D. Fenske and M. Mayor, Chem. – Eur. J., 2022, 28, e202201764 CAS; (g) M. Hasegawa, Y. Ishida, H. Sasaki, S. Ishioka, K. Usui, N. Hara, M. Kitahara, Y. Imai and Y. Mazaki, Chem. – Eur. J., 2021, 27, 16225 CrossRef CAS PubMed; (h) M. Tsuchiya, R. Inoue, K. Tanaka and Y. Morisaki, Chem. – Asian J., 2022, 17, e202200418 CrossRef CAS PubMed.
- (a) L. Kauffman, Topology, 1987, 26, 395 CrossRef; (b) L. Kauffman, Trans. Amer. Math. Soc., 1989, 311, 697 CrossRef.
- L. Bondarenko, I. Dix, H. Hinrichs and H. Hopf, Synthesis, 2004, 16, 2751 Search PubMed.
- (a) J. Kumar, T. Nakashima and T. Kawai, J. Phys. Chem. Lett., 2015, 6, 3445–3452 CrossRef CAS PubMed; (b) E. M. Sánchez-Carnerero, A. R. Agarrabeitia, F. Moreno, B. L. Maroto, G. Muller, M. J. Ortiz and S. de la Moya, Chem. – Eur. J., 2015, 21, 13488–13500 CrossRef PubMed; (c) J. Roose, B.-Z. Tang and K.-S. Wong, Small, 2016, 12, 6495–6512 CrossRef CAS PubMed; (d) M. Li, W.-B. Lin, L. Fang and C.-F. Chen, Acta Chim. Sin., 2017, 75, 1150–1163 CrossRef; (e) J. Han, S. Guo, H. Lu, S. Liu, Q. Zhao and W. Huang, Adv. Opt. Mater., 2018, 6, 1800538 CrossRef; (f) H. Tanaka, Y. Inoue and T. Mori, ChemPhotoChem, 2018, 2, 386–402 CrossRef CAS; (g) D. Zheng, L. Zheng, C. Yu, Y. Zhan, Y. Wang and H. Jiang, Org. Lett., 2019, 21, 2555–2559 CrossRef CAS PubMed; (h) D. Zheng, C. Yu, L. Zheng, Y. Zhan and H. Jiang, Chin. Chem. Lett., 2020, 31, 673–676 CrossRef CAS; (i) F. Song, Z. Zhao, Z. Liu, J. W. Y. Lam and B.-Z. Tang, J. Mater. Chem. C, 2020, 8, 3284–3301 RSC; (j) Circularly Polarized Luminescence of Isolated Small Organic Molecules, ed. T. Mori, Springer, Singapore, 2020 Search PubMed.
- (a) L. E. Perret-Aebi, A. von Zelewsky, C. D. Dietrich-Buchecker and J.-P. Sauvage, Angew. Chem., Int. Ed., 2004, 43, 4482 CrossRef CAS PubMed; (b) A. Theil, C. Mauve, M.-T. Adeline, A. Marinetti and J.-P. Sauvage, Angew. Chem., Int. Ed., 2006, 45, 2104 CrossRef CAS PubMed; (c) M. Feigel, R. Ladberg, S. Engels, R. Herbst-Irmer and R. Fröhlich, Angew. Chem., Int. Ed., 2006, 45, 5698 CrossRef CAS PubMed; (d) N. Ponnuswamy, F. B. L. Cougnon, J. M. Clough, G. D. Pantos and J. K. M. Sanders, Science, 2012, 338, 783 CrossRef CAS PubMed.
- (a) H. L. Schmider and A. D. Becke, Chemical content of the kinetic energy density, J. Mol. Struct.: THEOCHEM, 2000, 51–61 CrossRef CAS; (b) J. F. Gonthier, S. N. Steinmann, L. Roch, A. Ruggi, N. Luisier, K. Severin and C. Corminboeuf, Chem. Commun., 2012, 48, 9239 RSC; (c) Z. Liu, T. Lu, S. Hua and Y. Yu, J. Phys. Chem. C, 2019, 123, 18593 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2173517 and 2173519. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2sc06825g |
| This journal is © The Royal Society of Chemistry 2023 |