Open Access Article
Open Access ArticleFacile in situ formation of luminescent cellulose paper using Schweizer's reagent as an inorganic solvent in water†
Stephanie A.
Fraser
 ,
Michael N.
Pillay
and
Werner E.
van Zyl
,
Michael N.
Pillay
and
Werner E.
van Zyl
 *
*
School of Chemistry and Physics, University of KwaZulu-Natal, Westville Campus, Durban 4000, South Africa. E-mail: vanzylw@ukzn.ac.za
First published on 2nd July 2020
Abstract
A method to partially dissolve cellulose by an inorganic solvent, followed by in situ chemical reactions performed on the solvent, is described for the first time. The resulting composite formed a stable and luminescent Cu(I) cluster-functionalized cellulose paper with improved homogeneity in aqueous and alcoholic environments. The general synthesis methodology offers broad scope for further investigations.
Cellulose is formed by a wide variety of organisms such as plants, tunicates, fungi and bacteria, and is regarded as the most abundant biopolymer and renewable material on earth.1–4 The polymer is renowned for its biodegradability, non-toxicity and biocompatibility.5–7 In light of the current movement towards more sustainable technologies, these properties make cellulose an attractive choice for the design of high-performance future materials. To date, cellulose has already found widespread application ranging from tissue engineering to solar cells.8,9
Despite its numerous applications, the lack of suitable solvents to process cellulose is a major challenge and thus limits the amount of manufacturing.10,11 There is ongoing development for cheaper, environment-friendly, and non-degrading solvent systems for the complete and direct dissolution of cellulose under ambient conditions. Many common solvents are good swelling agents for cellulose but none of them manage to dissolve it. Suitable solvents for cellulose fall in two broad categories: derivatizing solvents and direct or non-derivatizing solvents. A derivatizing solvent induces covalent modification of the cellulose backbone rendering a derivative of cellulose soluble, not the native material. For example, the viscose (rayon) process – first described in 1893, is one of the most successful routes to dissolving and shaping cellulose forming cellulose xanthogenate with cellulose/CS2/NaOH components.12 Direct or non-derivatizing solvents form strong Coulombic interactions between the solvent and cellulose structure, without derivatizing it, which is preferred in analytical applications. These solvents also simplify production by minimizing reaction steps and are easier to recycle since less or no byproducts are generated. Graenacher pioneered a series of cellulose solutions that was patented13a,b already in 1934 and 1939 and that laid the foundation for what would become the Lyocell process, and makes particularly good use of the polar N–O bond in the solvent N-methylmorpholine-N-oxide (NMMO). In 1982, Chanzy et al. studied the crystallization and melting performance of the NMMO–H2O–cellulose system.13c The phase diagram-related cellulose/solvent systems include DMF/N2O4 or DMSO/N2O4, and HCOOH/H2SO4.14 Other widely used bicomponent solvent systems are dimethylacetate (DMAc)/LiCl,15,16 and DMSO/TBAF.17 Ionic liquids have also become a major role player in the dissolution of cellulose.18–20 In either a derivatizing or non-derivatizing solvent, the final step typically entails precipitation in an acid, for simultaneous neutralization and regeneration of the cellulose in a wet spinning step.
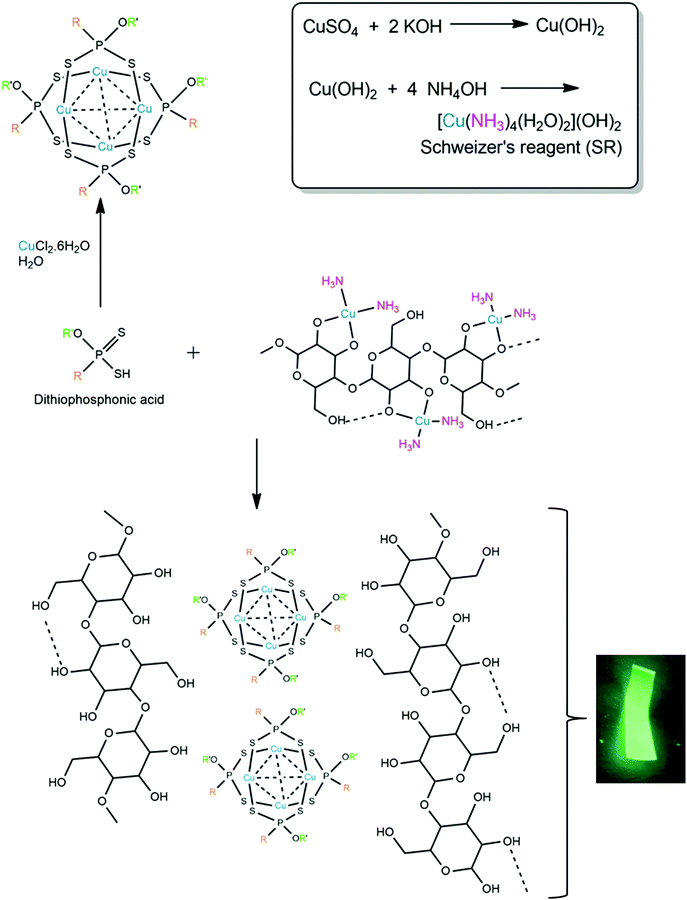
An interesting number of metal complexes have been successfully utilized as inorganic solvents for the dissolution of cellulose.21–24 The Cupro process, using cuprammonium hydroxide as solvent, is known since 1857 and is still used today,25,26 although not on large scale, presumably due to the higher cost of metal and separation processes involved. Such ammine complexes contain the metals Cd, Co, Ni, Pd, Zn and especially Cu that have been the most studied as Cuam or Cuoxam, also known as Schweizer's Reagent (SR), [Cu(NH3)4](OH)2.
In 1994, Burchard et al. laid the groundwork for the use of SR as a solvent for cellulose.27 They proposed that the dissolution mechanism involves the chelation of the deprotonated O-donor atoms of the cellulose monomers, while displacing two ammine ligands from the copper(II) complex (Scheme 1). The approach we followed was firstly to recognize the partial dissolution of cellulose by SR, where a Cu(II) ammine species complexes with the hydroxy groups of cellulose. This material remains stable as long as the solution remains basic (pH ≫ 7) and, importantly, the cellulose precipitates out of solution in the presence of any mono-, di- or triprotic acid. Typically, the conjugate base of the acid becomes the anion of the removed Cu(II) species, and in the present study, simultaneously becomes the ligand to form the cluster.
We have investigated the chemistry of dithiophosphonic acids [HS2PR(OR′)] and its coordination chemistry with group 11 metals (Cu, Ag, Au) for the past two decades.28–32 The acids are relatively strong (pKa ∼ 2–3) and are capable of protonating the cellulose hydroxy group and removing the Cu(II). We have recently reported on the reaction between Cu(II) salt and the [S2PR(OR′)]− ligand in water in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio, forming a luminescent neutral Cu4L4 cluster, as shown in Scheme 1.33 The addition of acid to a Cu(II) source (obtained directly by forming SR) should therefore form the same luminescent complex and have a strong interaction with the cellulose backbone. As demonstrated here, this hypothesis worked. Additionally, the photoluminescence of Cu(I) complexes has been well established34 which is particularly useful for applications in photonics, such as OLEDs, and thus these complexes serve as an attractive means of adding functionality to composite materials.35
1 molar ratio, forming a luminescent neutral Cu4L4 cluster, as shown in Scheme 1.33 The addition of acid to a Cu(II) source (obtained directly by forming SR) should therefore form the same luminescent complex and have a strong interaction with the cellulose backbone. As demonstrated here, this hypothesis worked. Additionally, the photoluminescence of Cu(I) complexes has been well established34 which is particularly useful for applications in photonics, such as OLEDs, and thus these complexes serve as an attractive means of adding functionality to composite materials.35
Considering the stated favourable properties of cellulose, we became particularly interested in the preparation of cellulose/Cu(I) cluster composite paper. A simple method by which this can be achieved is to immobilise the complex in the matrix of the material by blending the solid-phase Cu(I) cluster with the liquid-phase matrix before drying. However, there are many drawbacks associated with this method. Chief among these is poor homogeneity when the two components are combined. This is due to the cluster's insolubility in water leading to poor dispersibility within the water-based cellulose matrix. In addition, the cellulose suspension has considerable viscosity which would make incorporation of the cluster on the cellulose fibers difficult to achieve. We therefore probed an alternative approach to yield better quality composite paper. The preparation and stability of Schweizer's reagent rely on the presence of an excess of hydroxide ions. The hydroxide ions in solution act as a base and extract the protons from the hydroxyl groups on the cellulose, which is what drives the formation of the copper/cellulose complex (i.e., ‘solvated cellulose’). Thus, the dissolution of cellulose can only occur in a strongly basic environment. It follows that, upon the addition of acid, the cellobiose units become protonated and dissociate from the copper centre, resulting in the precipitation of regenerate cellulose fibers. Addition of the acid also quenches the ammonia in the system which causes SR to revert to the insoluble salt, copper(II) hydroxide. Mineral acids are most commonly used for this step. However, the ditihiophosphonate ligand of the copper(I) cluster can also be isolated as a dithiophosphonic acid.30 Conveniently, Schweizer's reagent is also a copper-based solution. Hence, the dithiophosphonic acid should simultaneously react with the cellulose/SR chelate to reform the cellulose fibers and revert SR to Cu(OH)2, as well as form the tetranuclear Cu(I) cluster (Scheme 1).
This study developed a method by which Schweizer's reagent is used to partially dissolve native cellulose following regular protocols but then go further and act as a source of copper for the in situ preparation of a tetranuclear copper(I) cluster. Dithiophosphonic acid is used to simultaneously regenerate the cellulose fibers and precipitate the Cu(I) dithiophosphonate cluster. It is beyond the scope of the present study to speculate on the detailed nature of the interaction between the cellulose fibers and the cluster. However, the study does clearly demonstrate that because both the copper and cellulose source emerge from an aqueous solution through a bottom-up approach, the paper produced has more stable functionality and vastly improved homogeneity when compared to simply mixing the phases together in the solid state. Notably, all of the reactions performed by the present method occurred under aqueous, room temperature conditions and our approach also utilized and functionalises the SR solvent to a large extent.
In this study we compared the properties of two papers, F1 and F2. Paper F1 was prepared by partially dissolving bacterial cellulose (BC) in SR, followed by the addition of the dithiophosphonic acid. The resulting suspension was dialysed and dried to form a paper. Paper F2 was prepared by blending the pre-isolated copper(I) cluster with the bacterial cellulose suspension, followed by drying to form a paper. A reference sample of the Cu(I) cluster (Cu4L4) was also prepared in the absence of cellulose by simply adding the dithiophosphonic acid to SR and isolating the powder that formed. Characterisation of this reference sample was used to confirm that the newly proposed method does in fact synthesise the Cu(I) cluster.
From the SEM micrographs of F1 and F2, it is clearly observed that the two different methods by which these papers are prepared generate very distinctive morphologies. In micrograph A (Fig. S1, ESI†), the surface of F1 appears smooth and in micrograph B (Fig. S1, ESI†) F2 is found to be significantly more uneven in texture, with aggregation of the cluster in certain regions. Taking a closer look, it can also be seen that the means by which the cluster precipitates in the SR/cellulose system differs significantly from the cellulose-free system. In F2, the cluster appears to be somewhat crystalline due to the observation of plate-like formations in micrographs C and D (Fig. S2, ESI†). Moreover, this precipitate appears to be superficially deposited on the fibers of the matrix. While for F1, the cluster appeared to have precipitated in the form of amorphous and spherical particles, as observed in micrographs A and B (Fig. S2, ESI†). These aggregates also appeared to be more embedded between the cellulose fibers. This indicates that the precipitation of the cluster in situ with the solvated cellulose fibers allowed for better incorporation of the cluster both throughout the volume and across the surface of the cellulose matrix.
Elemental dispersive X-ray spectroscopy (EDX) was used to analyse the surface composition of the papers. The EDX spectra of the papers all indicate a high content of oxygen and carbon, typical of cellulose-based materials. In addition, the spectra also signal the presence of phosphorus, sulfur, and copper as a result of the incorporation of the copper cluster into these composites (Table S1, ESI†). These elements were also mapped onto SEM micrographs to determine the surface elemental distribution on the papers (Fig. S3 and S4, ESI†). The elemental mapping corroborates the previous observation that the cluster (containing sulfur in the ligand) is distributed more evenly across the surface of F1, while for F2 there is considerable aggregation.
The papers were further characterised by X-ray diffraction (XRD) analysis. From the diffractogram (Fig. 1), the characteristic peaks of cellulose I at 2θ = 15° and 22.5°, are present.36 The broad peak at 2θ = 15° corresponds to the [110] and [1−10] crystallographic planes. The peak at 2θ = 22.5° was attributed to the [200] crystal planes.37 This high-intensity peak was used to confirm the presence of native cellulose in the composite papers. A small share of cellulose II is also visible in the spectrum of F1. This is a result of the transformation of cellulose I to cellulose II that occurs after dissolution in SR and subsequent reprecipitation. The common peak at 2θ = 8.5° was used to confirm the presence of the tetranuclear copper(I) cluster in the composite papers.
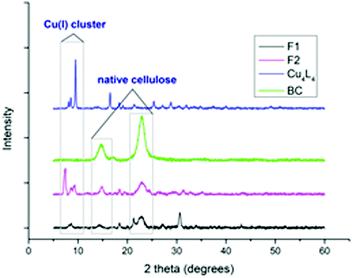 | ||
| Fig. 1 X-ray diffractograms of papers F1 and F2, Cu(I) cluster (Cu4L4) and bacterial cellulose (BC). | ||
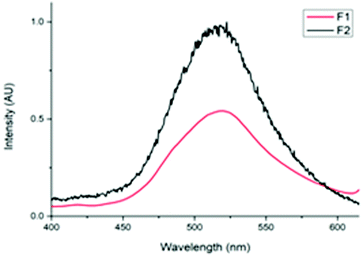
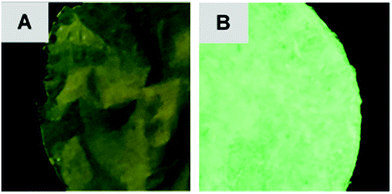
The photoluminescent (PL) nature of the papers was also investigated. Both F1 and F2 produced an emission peak at 519 nm (Fig. 2). This value was consistent with the data obtained previously for the pure solid-state tetranuclear copper(I) clusters. The broad, indistinguishable characteristics of these peaks are assigned to metal–ligand charge transfer (MLCT) emission with a contribution from the Cu⋯Cu cuprophilic interactions of the Cu atoms in the tetranuclear core (3d → 3d transitions).33 However, from the photographs of these papers it can be observed that the luminescence emission intensity of F2 is much greater than that of F1 (Fig. 3).
This result correlates with the SEM observations. Since the cluster appears to be both more crystalline and predominantly deposited on the surface of F2, it follows that this material would display more intense photoluminescence. While for F1, the cluster was more embedded within the paper and thus a significant portion of the cluster particles would be shielded by the fibers in which they are enveloped, resulting in a less intense emission.
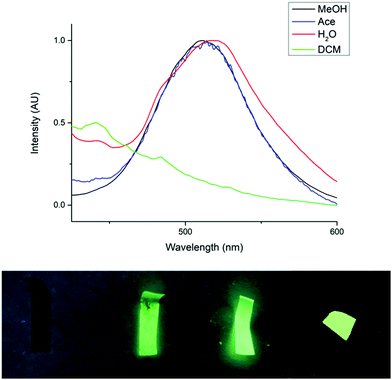
Further investigations were also carried out to determine the solvent stability of the novel paper, F1, in acetone, dichloromethane, methanol and water. In DCM, complete dissolution and detachment of the cluster from the cellulose paper was observed. This accounts for the absence of an emission peak at λ = 519 nm in the PL spectrum (Fig. 4). This result was not surprising since the copper cluster is completely soluble in DCM. Although the cluster was well embedded between the cellulose fibers, cellulose is highly permeable and thus we propose that the solvent would be able to penetrate the paper and dissolve the cluster. In acetone, partial removal of the cluster from the paper was observed. However, the paper was still mostly intact and the emission peak, although lower in intensity, was still observed. The exposure of the paper to methanol appeared to have had no effect, leaving the paper completely intact. F1 was also immersed in water for a period of two months and there was no observable detachment of the cluster. The stability of F1 in water is obviously very useful for potential future applications of these materials in humid or wet environments. The high stability of F1 in aqueous and alcoholic environments may be a result of two factors. Firstly, the fact that the cluster co-precipitates with the cellulose fibers and becomes entangled in this matrix makes it physically more difficult for the cluster to be washed away by polar solvents. Secondly, there may be additional interactions between the cellulose and the cluster, such as hydrogen bonding or Coulombic attraction, which may provide additional stability to these composites in solvents which cannot overcome these interactions.
Conclusions
We have demonstrated for the first time the preparation of functional regenerate cellulose paper by performing a chemical reaction on the inorganic solvent itself, in particular, the addition of dithiophosphonic acids to cellulose partially dissolved by Schweizer's reagent. This material possesses superior homogeneity and improved incorporation of the copper(I) cluster between the cellulose fibers compared to those prepared by superficially blending the two components. Moreover, we have shown that the paper prepared by this co-precipitation approach is highly stable in aqueous and alcoholic environments. This novel strategy serves as a facile method by which other inorganic metal complexes (Cu, Cd, Ni Co, etc.) with acidic ligand derivatives can potentially be incorporated into a cellulose-based composite, and in addition to optical properties exploited here, also be applied in the areas of catalysis and possibly biomaterials.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
The authors acknowledge support from the University of KwaZulu-Natal, financial support from the ESKOM TESP programme, and the National Research Foundation (NRF) for both an Honours and MSc degree bursary to S. A. F.References
- C. N. F. Vasconcelos, J. P. A. Feitosa, F. M. P. da Gama, J. P. S. Morais, F. K. Andrade, M. d. S. M. de Souza Filho and M. d. F. Rosa, Carbohydr. Polym., 2017, 155, 425–431 CrossRef PubMed.
- V. K. Varshney and S. Naithani, in Cellulose Fibers: Bio- and Nano-Polymer Composites: Green Chemistry and Technology, ed. S. Kalia, B. S. Kaith and I. Kaur, Springer, Berlin Heidelberg, 2011, pp. 43–60 Search PubMed.
- M. J. Dunlop, B. Acharya and R. Bissessur, J. Environ. Chem. Eng., 2018, 6, 4408–4412 CrossRef CAS.
- D. Klemm, B. Heublein, H.-P. Fink and A. Bohn, Angew. Chem., Int. Ed., 2005, 44, 3358–3393 CrossRef CAS PubMed.
- D. Klemm, E. D. Cranston, D. Fischer, M. Gama, S. A. Kedzior, D. Kralisch, F. Kramer, T. Kondo, T. Lindström, S. Nietzsche, K. Petzold-Welcke and F. Rauchfuß, Mater. Today, 2018, 21, 720–748 CrossRef CAS.
- J. George and S. N. Sabapathi, Nanotechnol., Sci. Appl., 2015, 8, 45–54 CrossRef CAS PubMed.
- A. Dufresne, Mater. Today, 2013, 16, 220–227 CrossRef CAS.
- P. Gatenholm and D. Klemm, MRS Bull., 2010, 35, 208–213 CrossRef CAS.
- F. Bella, S. Galliano, M. Falco, G. Viscardi, C. Barolo, M. Grätzel and C. Gerbaldi, Green Chem., 2017, 19, 1043–1051 RSC.
- B. Medronho, A. Romano, M. Gracia Miguel, L. Stigsson and B. Lindman, Cellulose, 2012, 19, 581–587 CrossRef CAS.
- M. Ghasemi, M. Tsianou and P. Alexandridis, Agri. Res. Tech.: Open Access J., 2018, 16, 555985 Search PubMed.
- F. Cross, E. T. Bevan and C. Beadle, Ber. Dtsch. Chem. Ges., 1893, 26, 1090–1097 CrossRef.
- (a) C. Graenacher, Cellulose solution, US Pat., 1943176, 1934 Search PubMed; (b) C. Graenacher and R. Sallmann, Cellulose solutions and process of making same, US Pat., 2179181, 1939 Search PubMed; (c) H. Chanzy, S. Nawrot, A. Peguy, P. Smith and J. Chevalier, J. Polym Sci. B, 1982, 20, 1909–1924 CAS.
- C. Olsson and G. Westman, Direct Dissolution of Cellulose: Background, Means and Applications, in: Cellulose – fundamental aspects, ed. T. G. M. van de Ven and L. Godbout, IntechOpen, 2013, ch. 6 Search PubMed.
- A. Striegel, Carbohydr. Polym., 1997, 34, 267–274 CrossRef CAS.
- C. L. McCormick and P. A. Callais, Polymer, 1987, 28, 2317–2323 CrossRef CAS.
- T. Heinze and S. Köhler, Dimethyl Sulfoxide and Ammonium Fluorides Novel Cellulose Solvents. In: Cellulose Solvents: For Analysis, Shaping and Chemical Modification. American Chemical Society, 2020, pp. 103–118 Search PubMed.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974–4975 CrossRef CAS PubMed.
- G. Viswanathan, S. Murugesan, V. Pushpraj, O. Nalamasu, P. M. Ajayan and R. J. Linhardt, Biomacromolecules, 2006, 7, 415–418 CrossRef CAS PubMed.
- B. Kosan, C. Michels and F. Meister, Cellulose, 2008, 15, 59–66 CrossRef CAS.
- T. Heinze and T. Liebert, Prog. Polym. Sci., 2001, 26, 1689–1762 CrossRef CAS.
- K. Saalwächter, W. Burchard, P. Klüfers, G. Kettenbach, P. Mayer, D. Klemm and S. Dugarmaa, Macromolecules, 2000, 33, 4094–4107 CrossRef.
- R. Ahlrichs, M. Ballauff, K. Eichkorn, O. Hanemann, G. Kettenbach and P. Klüfers, Chem. – Eur. J., 1998, 4, 835–844 CrossRef CAS.
- O. Hanemann and M. Ballauff, Macromolecules, 1997, 30, 7638–7640 CrossRef CAS.
- C. Woodings, Regenerated Cellulose Fibres, Woodhead publishing Ltd., Cambridge, 2001 Search PubMed.
- E. Schweizer, J Prukt. Chem., 1857, 72, 109–111 CrossRef.
- W. Burchard, N. Habermann, P. Klüfers, B. Seger and U. Wilhelm, Angew. Chem., Int. Ed. Engl., 1994, 33, 884–887 CrossRef.
- W. E. van Zyl, Comments Inorg. Chem., 2010, 31, 13–45 CrossRef CAS.
- W. E. van Zyl and J. D. Woollins, Coord. Chem. Rev., 2013, 257, 718–731 CrossRef CAS.
- W. E. van Zyl and J. P. Fackler, Jr., Phosphorus, Sulfur Silicon Relat. Elem., 2000, 167, 117–132 CrossRef CAS.
- M. N. Pillay, B. Omondi, R. J. Staples and W. E. van Zyl, CrystEngComm, 2013, 15, 4417–4421 RSC.
- W. E. van Zyl, J. M. López-de-Luzuriaga, J. P. Fackler, Jr. and R. J. Staples, Can. J. Chem., 2001, 79, 896–903 CrossRef CAS.
- M. N. Pillay, J.-H. Liao, C. W. Liu and W. E. van Zyl, Inorg. Chem., 2019, 58, 7099–7106 CrossRef CAS PubMed.
- T. Hofbeck, U. Monkowius and H. Yersin, J. Am. Chem. Soc., 2015, 137, 399–404 CrossRef CAS PubMed.
- L. P. Ravaro, K. P. S. Zanoni and A. S. S. de Camargo, Energy Rep., 2019, 6, 37–45 CrossRef.
- S. Sheykhnazari, T. Tabarsa, A. Ashori, A. Shakeri and M. Golalipour, Carbohydr. Polym., 2011, 86, 1187–1191 CrossRef CAS.
- C. Borsoi, H. Ornaghi, L. Scienza, A. Zattera and C. A. Ferreira, Polym. Polym. Compos., 2017, 25, 563–570 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis methods, SEM and EDX micrographs, NMR spectra, video of reaction. See DOI: 10.1039/d0ma00135j |
| This journal is © The Royal Society of Chemistry 2020 |