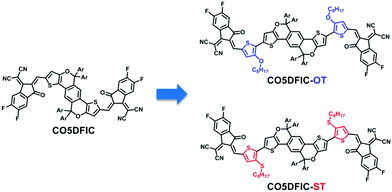
Alkoxythiophene and alkylthiothiophene π-bridges enhance the performance of A–D–A electron acceptors†
Lei
Zhang‡
ab,
Ke
Jin‡
b,
Zuo
Xiao
 b,
Xingzhu
Wang
*a,
Tao
Wang
b,
Xingzhu
Wang
*a,
Tao
Wang
 *c,
Chenyi
Yi
*d and
Liming
Ding
*c,
Chenyi
Yi
*d and
Liming
Ding
 *b
*b
aCollege of Chemistry, Xiangtan University, Xiangtan, Hunan 411105, China. E-mail: xzwang@xtu.edu.cn
bCenter for Excellence in Nanoscience (CAS), Key Laboratory of Nanosystem and Hierarchical Fabrication (CAS), National Center for Nanoscience and Technology, Beijing 100190, China. E-mail: ding@nanoctr.cn
cSchool of Materials Science and Engineering, Wuhan University of Technology, Wuhan 430070, China. E-mail: twang@whut.edu.cn
dDepartment of Electrical Engineering, Tsinghua University, Beijing 100084, China. E-mail: yicy@tsinghua.edu.cn
First published on 17th January 2019
Abstract
Three nonfullerene acceptors CO5DFIC, CO5DFIC-OT and CO5DFIC-ST were developed. CO5DFIC-OT and CO5DFIC-ST have alkoxythiophene and alkylthiothiophene π-bridges, respectively, and they show higher LUMO levels and enhanced light-harvesting capability compared to CO5DFIC without π-bridges. CO5DFIC-OT and CO5DFIC-ST solar cells gave higher open-circuit voltage, short-circuit current density and power conversion efficiency than CO5DFIC cells.
Recently, considerable progress has been made in organic solar cells due to the rapid development of efficient nonfullerene acceptors (NFAs).1 Compared with fullerene acceptors, NFAs have advantages like tunable energy levels, strong light-harvesting capability and good morphological stability.1a NFA solar cells offer high power conversion efficiencies (PCEs) (>14% for single-junction and >17% for tandem).2 Acceptor–donor–acceptor (A–D–A) small molecule acceptors are promising NFAs.3 A–D–A NFAs usually consist of a ladder-type electron-donating core unit and two electron-accepting end units. π-Bridges can be added between the core and end units to tune optoelectronic properties. A–D–A NFAs with π-bridges like thiophene,4 thieno[3,4-b]thiophene,5 benzo[c][1,2,5]thiadiazole6 and benzo[d][1,2,3]triazole7 have been reported. Alkoxythiophene (OT) and alkylthiothiophene (ST) are electron-rich π-bridges widely used for improving the performance of donor materials.8 S⋯O and S⋯S noncovalent interactions can lock the conformation, enhance molecular packing and facilitate charge transport for the donors.8 However, their application in A–D–A NFAs is less explored.9 In this work, we developed three A–D–A acceptors CO5DFIC, CO5DFIC-OT and CO5DFIC-ST, and studied the effect of OT and ST π-bridges on material properties and photovoltaic performance (Fig. 1). Both OT and ST can lift the lowest unoccupied molecular orbital (LUMO) levels and improve the light-harvesting capability of A–D–A NFAs, thus enhancing the open-circuit voltage (Voc), short-circuit current density (Jsc) and PCE for solar cells. When blended with donor PTB7-Th, CO5DFIC-ST with ST π-bridges afforded a PCE of 9.73%, which is much higher than that of CO5DFIC (5.58%).
The synthetic routes to CO5DFIC, CO5DFIC-OT and CO5DFIC-ST are shown in Scheme S1 (ESI†). Core unit CO5 was prepared according to our previous report.1c OT and ST π-bridges were introduced via Stille coupling. The difluoro-substituted 1,1-dicyanomethylene-3-indanone (DFIC) end units were introduced via Knoevenagel condensation. The details are given in the ESI.† Intermediates and the final NFAs were characterized by nuclear magnetic resonance (NMR) and mass spectroscopy (see the ESI†). These molecules show good solubility in common solvents such as chloroform, toluene and chlorobenzene.
The absorption spectra of CO5DFIC, CO5DFIC-OT and CO5DFIC-ST in chloroform and as films are shown in Fig. S15 (ESI†) and Fig. 2a, respectively. In solution, they show a strong intramolecular charge transfer (ICT) absorption band, with a peak at 684 nm, 811 nm and 753 nm, respectively (Table 1). For films, the spectra show bathochromic shifts. From solution to films, the redshifts for CO5DFIC, CO5DFIC-OT and CO5DFIC-ST are 35 nm, 52 nm and 67 nm, respectively. The larger redshifts for CO5DFIC-OT and CO5DFIC-ST than that of CO5DFIC suggest that OT and ST π-bridges favor molecular packing in the solid state. The absorption onsets for CO5DFIC, CO5DFIC-OT and CO5DFIC-ST films are 794 nm, 972 nm and 928 nm, respectively, corresponding to optical bandgaps (Eoptg) of 1.56 eV, 1.28 eV and 1.34 eV, respectively. Smaller Eoptg values of CO5DFIC-OT and CO5DFIC-ST suggest that the electron-rich OT and ST π-bridges strengthen the ICT, leading to a bandgap shrink. Meanwhile, the ICT bands of CO5DFIC-OT and CO5DFIC-ST are broader than that of CO5DFIC. The FWHM are 148 nm, 206 nm and 209 nm for CO5DFIC, CO5DFIC-OT and CO5DFIC-ST, respectively. The smaller Eoptg and broadened ICT bands demonstrate the improved light-harvesting capability of CO5DFIC-OT and CO5DFIC-ST, which could bring high Jsc for solar cells. Compared with the spectrum of CO5DFIC, the spectra of CO5DFIC-OT and CO5DFIC-ST are more complementary with that of donor PTB7-Th (absorption band at 500–800 nm).
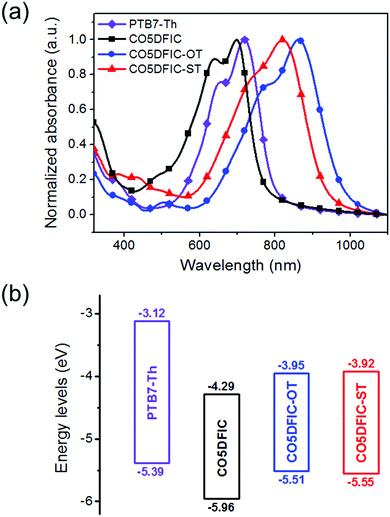 | ||
| Fig. 2 (a) Absorption spectra of PTB7-Th, CO5DFIC, CO5DFIC-OT and CO5DFIC-ST films; and (b) their energy level diagrams. | ||
| Acceptors | λ sol [nm] | λ film [nm] | λ on [nm] |
E
optg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a [eV] a [eV] |
E onox [V] | E onred [V] | HOMOb [eV] | LUMOc [eV] |
E
ecg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d [eV] d [eV] |
|---|---|---|---|---|---|---|---|---|---|
| a E optg = 1240/λon. b HOMO = −(Eonox + 4.8). c LUMO = −(Eonred + 4.8). d E ecg = LUMO−HOMO. | |||||||||
| CO5DFIC | 684 | 719 | 794 | 1.56 | 1.16 | −0.51 | −5.96 | −4.29 | 1.67 |
| CO5DFIC-OT | 811 | 863 | 972 | 1.28 | 0.71 | −0.85 | −5.51 | −3.95 | 1.56 |
| CO5DFIC-ST | 753 | 820 | 928 | 1.34 | 0.75 | −0.88 | −5.55 | −3.92 | 1.63 |
The energy levels of CO5DFIC, CO5DFIC-OT and CO5DFIC-ST were evaluated by cyclic voltammetry (CV) (Fig. S16, ESI†).10 The energy level diagram is given in Fig. 2b. The highest occupied molecular orbital (HOMO) levels of CO5DFIC, CO5DFIC-OT and CO5DFIC-ST are −5.96 eV, −5.51 eV and −5.55 eV, respectively, and the LUMO levels are −4.29 eV, −3.95 eV and −3.92 eV, respectively. The electrochemical bandgaps (Eecg) of CO5DFIC, CO5DFIC-OT and CO5DFIC-ST are 1.67 eV, 1.56 eV and 1.63 eV, respectively, which are close to Eoptg. The Eecg values are 0.1–0.3 eV higher than Eoptg, which might result from the interface barrier between the film and the electrode surface during CV measurements.11 OT and ST π-bridges lift both HOMO and LUMO levels of the materials. The higher LUMO levels of CO5DFIC-OT and CO5DFIC-ST are favorable for obtaining higher Voc in solar cells. Donor PTB7-Th has a HOMO at −5.39 eV and a LUMO at −3.12 eV.1e
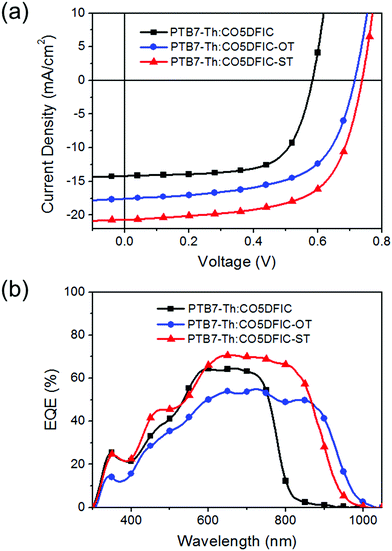
Inverted solar cells with a structure of ITO/ZnO/PTB7-Th:NFA/MoO3/Ag were made to evaluate the performance of CO5DFIC, CO5DFIC-OT and CO5DFIC-ST.12J–V curves and external quantum efficiency (EQE) spectra are shown in Fig. 3a and b, respectively, and the performance data are listed in Table 2. The best PTB7-Th:CO5DFIC cells gave a PCE of 5.58%, with a Voc of 0.58 V, a Jsc of 14.19 mA cm−2 and a fill factor (FF) of 67.4%. These cells have a D/A ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w), an active layer thickness of 90 nm and 3 vol% 1-chloronaphthalene (CN) as the additive (Tables S1–S3, ESI†). The best PTB7-Th:CO5DFIC-OT cells gave a PCE of 7.66%, with a Voc of 0.71 V, a Jsc of 17.58 mA cm−2 and a FF of 61.0%. These cells have a D/A ratio of 1
1 (w/w), an active layer thickness of 90 nm and 3 vol% 1-chloronaphthalene (CN) as the additive (Tables S1–S3, ESI†). The best PTB7-Th:CO5DFIC-OT cells gave a PCE of 7.66%, with a Voc of 0.71 V, a Jsc of 17.58 mA cm−2 and a FF of 61.0%. These cells have a D/A ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 (w/w), an active layer thickness of 85 nm and 3 vol% CN as the additive (Tables S4–S6, ESI†). The best PTB7-Th:CO5DFIC-ST cells gave a PCE of 9.73%, with a Voc of 0.74 V, a Jsc of 20.71 mA cm−2 and a FF of 63.7%. These cells have a D/A ratio of 1
1.5 (w/w), an active layer thickness of 85 nm and 3 vol% CN as the additive (Tables S4–S6, ESI†). The best PTB7-Th:CO5DFIC-ST cells gave a PCE of 9.73%, with a Voc of 0.74 V, a Jsc of 20.71 mA cm−2 and a FF of 63.7%. These cells have a D/A ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 (w/w), an active layer thickness of 102 nm and 3 vol% CN as the additive (Tables S7–S9, ESI†). Compared with CO5DFIC cells, CO5DFIC-OT and CO5DFIC-ST cells show higher performance due to higher Voc and Jsc. The higher Voc values for CO5DFIC-OT and CO5DFIC-ST cells are due to the higher LUMO levels of CO5DFIC-OT and CO5DFIC-ST, and the higher Jsc values originate from the stronger light-harvesting capability of CO5DFIC-OT and CO5DFIC-ST. CO5DFIC-OT and CO5DFIC-ST cells gave much broader EQE spectra than CO5DFIC cells (Fig. 3b). The integrated current densities obtained from EQE spectra for CO5DFIC, CO5DFIC-OT and CO5DFIC-ST cells are 13.78 mA cm−2, 16.46 mA cm−2 and 19.60 mA cm−2, respectively, which are consistent with the Jsc values obtained from J–V measurements. The maximum EQE (EQEmax) for CO5DFIC, CO5DFIC-OT and CO5DFIC-ST cells are 65%, 55% and 71%, respectively, suggesting that charge generation is more efficient in CO5DFIC-ST cells but less efficient in CO5DFIC-OT cells. Indeed, the exciton dissociation probabilities (Pdiss) for CO5DFIC, CO5DFIC-OT and CO5DFIC-ST cells are 91.5%, 87.5% and 95.3%, respectively, consistent with EQEmax (Fig. S17, ESI†).13 In contrast to Voc and Jsc, the FF decreased in CO5DFIC-OT and CO5DFIC-ST cells. To understand this, we measured hole and electron mobilities (μh and μe) of PTB7-Th:NFA blend films by using the space charge limited current (SCLC) method (Fig. S18 and S19, Table S10, ESI†).14 Compared with the PTB7-Th:CO5DFIC film, PTB7-Th:CO5DFIC-OT and PTB7-Th:CO5DFIC-ST films show higher μh but lower μe. The μh/μe values of PTB7-Th:CO5DFIC, PTB7-Th:CO5DFIC-OT and PTB7-Th:CO5DFIC-ST films are 1.8, 9.1 and 6.7, respectively. The unbalanced charge transport in CO5DFIC-OT and CO5DFIC-ST cells accounts for the lower FF. We also studied bimolecular recombination by plotting Jsc against light intensity (Plight) (Fig. S20, ESI†). The data were fitted to a power law: Jsc ∝ Pαlight. The α values of CO5DFIC, CO5DFIC-OT and CO5DFIC-ST cells are 99.6%, 95.9% and 98.6%, respectively, suggesting more bimolecular recombination in CO5DFIC-OT and CO5DFIC-ST cells,15 which also leads to a lower FF. The morphology of the active layers was studied by using an atomic force microscope (AFM) (Fig. S21, ESI†). The PTB7-Th:CO5DFIC film presents a coarser surface than PTB7-Th:CO5DFIC-OT and PTB7-Th:CO5DFIC-ST films. The root-mean-square (RMS) roughnesses are 3.67 nm, 1.86 nm and 2.69 nm, respectively. All blend films present clear nanostructures, suggesting good nanoscale phase separation.
2.5 (w/w), an active layer thickness of 102 nm and 3 vol% CN as the additive (Tables S7–S9, ESI†). Compared with CO5DFIC cells, CO5DFIC-OT and CO5DFIC-ST cells show higher performance due to higher Voc and Jsc. The higher Voc values for CO5DFIC-OT and CO5DFIC-ST cells are due to the higher LUMO levels of CO5DFIC-OT and CO5DFIC-ST, and the higher Jsc values originate from the stronger light-harvesting capability of CO5DFIC-OT and CO5DFIC-ST. CO5DFIC-OT and CO5DFIC-ST cells gave much broader EQE spectra than CO5DFIC cells (Fig. 3b). The integrated current densities obtained from EQE spectra for CO5DFIC, CO5DFIC-OT and CO5DFIC-ST cells are 13.78 mA cm−2, 16.46 mA cm−2 and 19.60 mA cm−2, respectively, which are consistent with the Jsc values obtained from J–V measurements. The maximum EQE (EQEmax) for CO5DFIC, CO5DFIC-OT and CO5DFIC-ST cells are 65%, 55% and 71%, respectively, suggesting that charge generation is more efficient in CO5DFIC-ST cells but less efficient in CO5DFIC-OT cells. Indeed, the exciton dissociation probabilities (Pdiss) for CO5DFIC, CO5DFIC-OT and CO5DFIC-ST cells are 91.5%, 87.5% and 95.3%, respectively, consistent with EQEmax (Fig. S17, ESI†).13 In contrast to Voc and Jsc, the FF decreased in CO5DFIC-OT and CO5DFIC-ST cells. To understand this, we measured hole and electron mobilities (μh and μe) of PTB7-Th:NFA blend films by using the space charge limited current (SCLC) method (Fig. S18 and S19, Table S10, ESI†).14 Compared with the PTB7-Th:CO5DFIC film, PTB7-Th:CO5DFIC-OT and PTB7-Th:CO5DFIC-ST films show higher μh but lower μe. The μh/μe values of PTB7-Th:CO5DFIC, PTB7-Th:CO5DFIC-OT and PTB7-Th:CO5DFIC-ST films are 1.8, 9.1 and 6.7, respectively. The unbalanced charge transport in CO5DFIC-OT and CO5DFIC-ST cells accounts for the lower FF. We also studied bimolecular recombination by plotting Jsc against light intensity (Plight) (Fig. S20, ESI†). The data were fitted to a power law: Jsc ∝ Pαlight. The α values of CO5DFIC, CO5DFIC-OT and CO5DFIC-ST cells are 99.6%, 95.9% and 98.6%, respectively, suggesting more bimolecular recombination in CO5DFIC-OT and CO5DFIC-ST cells,15 which also leads to a lower FF. The morphology of the active layers was studied by using an atomic force microscope (AFM) (Fig. S21, ESI†). The PTB7-Th:CO5DFIC film presents a coarser surface than PTB7-Th:CO5DFIC-OT and PTB7-Th:CO5DFIC-ST films. The root-mean-square (RMS) roughnesses are 3.67 nm, 1.86 nm and 2.69 nm, respectively. All blend films present clear nanostructures, suggesting good nanoscale phase separation.
| D:A | V OC [V] | J SC [mA cm−2] | FF [%] | PCE [%] |
|---|---|---|---|---|
| a The data in the parentheses are integrated current densities from EQE spectra. b The data in the parentheses are averages for 10 cells. | ||||
| PTB7-Th:CO5DFIC | 0.58 | 14.19 (13.78)a | 67.4 | 5.58 (5.37)b |
| PTB7-Th:CO5DFIC-OT | 0.71 | 17.58 (16.46)a | 61.0 | 7.66 (7.37)b |
| PTB7-Th:CO5DFIC-ST | 0.74 | 20.71 (19.60)a | 63.7 | 9.73 (9.37)b |
Conclusions
The electron-rich alkoxythiophene (OT) and alkylthiothiophene (ST) π-bridges were used to develop efficient A–D–A NFAs. The two acceptors, CO5DFIC-OT and CO5DFIC-ST, present higher LUMO levels and enhanced light-harvesting capability compared to CO5DFIC without π-bridges. CO5DFIC-OT and CO5DFIC-ST solar cells gave higher Voc, Jsc and PCEs than CO5DFIC cells. A 9.73% efficiency was obtained from PTB7-Th:CO5DFIC-ST cells. The results suggest that OT and ST π-bridges are useful in developing high-performance A–D–A acceptors. Further studies on design and synthesis are ongoing with the goal to improve the photocurrent.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key Research and Development Program of China (2017YFA0206600), the National Natural Science Foundation of China (U1401244, 21572041, 51503050, 51773045, 21772030, 21704021, 51473139 and 20974091) and the Youth Association for Promoting Innovation (CAS). X. Wang also acknowledges the Innovation Platform Open Foundation of University of Hunan Province (14K092) and the Hunan 2011 Collaborative Innovation Center of Chemical Engineering & Technology for financial support.References
- (a) C. Yan, S. Barlow, Z. Wang, H. Yan, A. K.-Y. Jen, S. R. Marder and X. Zhan, Nat. Rev. Mater., 2018, 3, 18003 CrossRef CAS; (b) Z. Xiao, S. Yang, Z. Yang, J. Yang, H.-L. Yip, F. Zhang, F. He, T. Wang, J. Wang, Y. Yuan, H. Yang, M. Wang and L. Ding, Adv. Mater., 2018 DOI:10.1002/adma.201804790; (c) Z. Xiao, F. Liu, X. Geng, J. Zhang, S. Wang, Y. Xie, Z. Li, H. Yang, Y. Yuan and L. Ding, Sci. Bull., 2017, 62, 1331 CrossRef CAS; (d) Z. Xiao, X. Jia, D. Li, S. Wang, X. Geng, F. Liu, J. Chen, S. Yang, T. P. Russell and L. Ding, Sci. Bull., 2017, 62, 1494 CrossRef CAS; (e) Z. Xiao, X. Jia and L. Ding, Sci. Bull., 2017, 62, 1562 CrossRef CAS; (f) T. Li, H. Zhang, Z. Xiao, J. J. Rech, H. Niu, W. You and L. Ding, Mater. Chem. Front., 2018, 2, 700 RSC; (g) Q. Liu, Z. Xiao, T. Li, S. Yang, W. You, M. Wang and L. Ding, Mater. Chem. Front., 2018, 2, 1563 RSC; (h) K. Jin, C. Deng, L. Zhang, D. Li, T. Li, F. Wang, Y. Yuan, Z. Xiao and L. Ding, Mater. Chem. Front., 2018, 2, 1716 RSC; (i) M. Zhang, Z. Xiao, W. Gao, Q. Liu, K. Jin, W. Wang, Y. Mi, Q. An, X. Ma, X. Liu, C. Yang, L. Ding and F. Zhang, Adv. Energy Mater., 2018, 8, 1801968 CrossRef.
- (a) H. Li, Z. Xiao, L. Ding and J. Wang, Sci. Bull., 2018, 63, 340 CrossRef CAS; (b) L. Meng, Y. Zhang, X. Wan, C. Li, X. Zhang, Y. Wang, X. Ke, Z. Xiao, L. Ding, R. Xia, H.-L. Yip, Y. Cao and Y. Chen, Science, 2018, 361, 1094 CrossRef CAS PubMed.
- Y. Lin, J. Wang, Z. Zhang, H. Bai, Y. Li, D. Zhu and X. Zhan, Adv. Mater., 2015, 27, 1170 CrossRef CAS PubMed.
- L. Yang, M. Li, J. Song, Y. Zhou, Z. Bo and H. Wang, Adv. Funct. Mater., 2018, 28, 170592 Search PubMed.
- F. Liu, Z. Zhou, C. Zhang, T. Vergote, H. Fan, F. Liu and X. Zhu, J. Am. Chem. Soc., 2016, 138, 15523 CrossRef CAS PubMed.
- D. Baran, T. Kirchartz, S. Wheeler, S. Dimitrov, M. Abdelsamie, J. Gorman, R. S. Ashraf, S. Holliday, A. Wadsworth, N. Gasparini, P. Kaienburg, H. Yan, A. Amassian, C. J. Brabec, J. R. Durrant and I. McCulloch, Energy Environ. Sci., 2016, 9, 3783 RSC.
- A. Tang, B. Xiao, Y. Wang, F. Gao, K. Tajima, H. Bin, Z.-G. Zhang, Y. Li, Z. Wei and E. Zhou, Adv. Funct. Mater., 2018, 28, 1704507 CrossRef.
- (a) S. Shi, Q. Liao, Y. Tang, H. Guo, X. Zhou, Y. Wang, T. Yang, Y. Liang, X. Cheng, F. Liu and X. Guo, Adv. Mater., 2016, 28, 9969 CrossRef CAS PubMed; (b) J. Chen, Z. Yan, L. Tang, M. A. Uddin, J. Yu, X. Zhou, K. Yang, Y. Tang, T. J. Shin, H. Woo and X. Guo, Macromolecules, 2018, 51, 5352 CrossRef CAS; (c) J. Chen, Q. Liao, G. Wang, Z. Yan, H. Wang, Y. Wang, X. Zhang, Y. Tang, A. Facchetti, T. J. Marks and X. Guo, Macromolecules, 2018, 51, 3874 CrossRef CAS; (d) L. Zhang, N. S. Colella, B. P. Cherniawski, S. C. B. Mannsfeld and A. L. Briseno, ACS Appl. Mater. Interfaces, 2014, 6, 5327 CrossRef CAS PubMed; (e) Z. Zhang, Z. Lu, J. Zhang, Y. Liu, S. Feng, L. Wu, R. Hou, X. Xu and Z. Bo, Org. Electron., 2017, 40, 36 CrossRef CAS; (f) Y. Zou, Y. Wu, H. Yang, Y. Dong, C. Cui and Y. Li, Mol. Syst. Des. Eng., 2018, 3, 131 RSC; (g) D. Yang, T. Zhang, X. Zhao, G. Zeng, Z. Li, Y. Tian, F. He, J. Zhang and X. Yang, Polym. Chem., 2016, 7, 5366 RSC.
- P. Chen, J. Wang, Q. Zhang, W. Huang, J. Zhu, R. Wang, S.-Y. Chang, P. Sun, L. Meng, H. Zhao, H.-W. Cheng, T. Huang, Y. Liu, C. Wang, C. Zhu, W. You, X. Zhan and Y. Yang, Adv. Mater., 2018, 30, 1801501 CrossRef PubMed.
- Z. Xiao, G. Ye, Y. Liu, S. Chen, Q. Peng, Q. Zuo and L. Ding, Angew. Chem., Int. Ed., 2012, 51, 9038 CrossRef CAS PubMed.
- W. Zhang, J. Cao, Y. Liu, Z. Xiao, W. Zhu, Q. Zuo and L. Ding, Macromol. Rapid Commun., 2012, 33, 1574 CrossRef CAS PubMed.
- Z. Xiao, X. Geng, D. He, X. Jia and L. Ding, Energy Environ. Sci., 2016, 9, 2114 RSC.
- D. Li, Z. Xiao, S. Wang, X. Geng, S. Yang, J. Fang, H. Yang and L. Ding, Adv. Energy Mater., 2018, 8, 1800397 CrossRef.
- M. An, F. Xie, X. Geng, J. Zhang, J. Jiang, Z. Lei, D. He, Z. Xiao and L. Ding, Adv. Energy Mater., 2017, 7, 1602509 CrossRef.
- H. Li, J. Cao, Q. Zhou, L. Ding and J. Wang, Nano Energy, 2015, 15, 125 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Preparation and characterization of materials, fabrication and measurements of solar cells. See DOI: 10.1039/c8qm00647d |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2019 |