Themed collection Novel π-electron molecular scaffolds

Novel π-electron molecular scaffolds: a themed issue
Novel π-electron molecular scaffolds with exciting properties will bring new discussions and excitement to the chemical community.

Org. Chem. Front., 2017,4, 648-649
https://doi.org/10.1039/C7QO90019H
Nanocalipers as novel molecular scaffolds for carbon nanotubes
Nanocalipers were synthesized by connecting directly the five aromatic moieties including two receptors, two corners and a core, and found to discriminate the diameter, metallicity and handedness of carbon nanotubes through selective complexation.
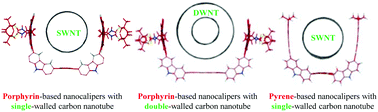
Org. Chem. Front., 2017,4, 911-919
https://doi.org/10.1039/C7QO00158D
Methodology and applications of the hexadehydro-Diels–Alder (HDDA) reaction
Hexadehydro-Diels–Alder (HDDA) reactions between alkynes and 1,3-diynes readily generate highly reactive and synthetically useful arynes.
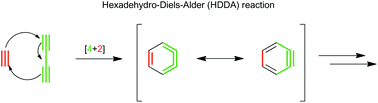
Org. Chem. Front., 2017,4, 891-910
https://doi.org/10.1039/C7QO00071E
Pentadecaphenylenes: synthesis, self-assembly and complexation with fullerene C60
Macrocyclic pentadecaphenylene incorporates fullerene C60 in its cavity to afford fibrous 2 : 1 and 1 : 1 sandwich complexes.
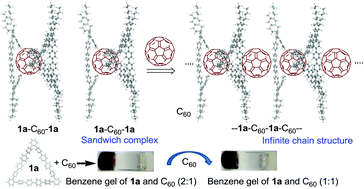
Org. Chem. Front., 2017,4, 882-890
https://doi.org/10.1039/C7QO00258K
6,6′,11,11′-Tetra((triisopropylsilyl)ethynyl)-anti-[2.2](1,4)tetracenophane: a covalently coupled tetracene dimer and its structural, electrochemical, and photophysical characterization
[2.2]-Acenophanes are a class of compounds with two acene units interconnected by two ethano bridges.
tetracenophane: a covalently coupled tetracene dimer and its structural, electrochemical, and photophysical characterization](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C7QO00117G&imageInfo.ImageIdentifier.Year=2017)
Org. Chem. Front., 2017,4, 853-860
https://doi.org/10.1039/C7QO00117G
Pyrene-fused bisphenazinothiadiazoles with red to NIR electroluminescence
Deep red and NIR electroluminescence from pyrene-fused bisphenazinothiadiazoles.
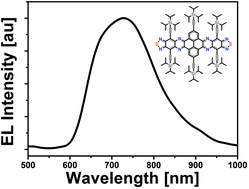
Org. Chem. Front., 2017,4, 876-881
https://doi.org/10.1039/C7QO00227K
Synthesis of 2,3-diaza-anthraquinones via the bidentate Lewis acid catalysed inverse electron-demand Diels–Alder (IEDDA) reaction
A new synthesis of aza-anthraquinones and substituted anthraquinones via bidentate Lewis acid catalysis has been developed.
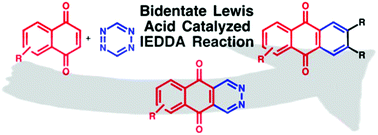
Org. Chem. Front., 2017,4, 871-875
https://doi.org/10.1039/C7QO00172J
Planar versus triptycenylene end-capped aroyleneimidazoles as electron acceptors in organic photovoltaics
The effect of triptycenylene end-groups on the optoelectronic properties of aroyleneimidazoles and their performance as acceptors in bulk heterojunction photovoltaic devices are described.
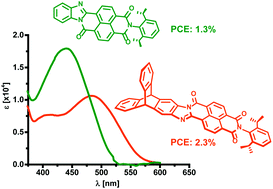
Org. Chem. Front., 2017,4, 834-838
https://doi.org/10.1039/C7QO00231A
Polycyclic heteroaromatic hydrocarbons containing a benzoisoindole core
By the combination of 9a-azaphenalene and a perpendicularly oriented acene, we have synthesized three derivatives of a series of novel, fully-conjugated nitrogen-containing polycyclic aromatic hydrocarbons (PAHs).
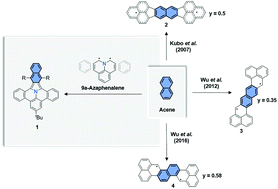
Org. Chem. Front., 2017,4, 847-852
https://doi.org/10.1039/C7QO00180K
Radical cation and dication of a 4H-dithieno[2,3-b:3′,2′-e][1,4]-thiazine
4H-Dithieno[2,3-b:3’,2’-e][1,4]-thiazine (DTT), and its radical cation and dication were synthesized, characterized (EPR spectroscopy and spectroelectrochemistry) and interpreted (DFT and TD DFT calculations).
![Graphical abstract: Radical cation and dication of a 4H-dithieno[2,3-b:3′,2′-e][1,4]-thiazine](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C7QO00188F&imageInfo.ImageIdentifier.Year=2017)
Org. Chem. Front., 2017,4, 839-846
https://doi.org/10.1039/C7QO00188F
Unexpected formation of [5]helicenes from hexaarylbenzenes containing pyrrole moieties
Surprisingly helical: a pyrrolic [5]helicene embedded into a six-membered ring framework is preferred over a heterocyclic pendant of hexabenzocoronene.
![Graphical abstract: Unexpected formation of [5]helicenes from hexaarylbenzenes containing pyrrole moieties](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C7QO00112F&imageInfo.ImageIdentifier.Year=2017)
Org. Chem. Front., 2017,4, 861-870
https://doi.org/10.1039/C7QO00112F
Synthesis of largely π-extended naphthalenediimides via C–H activation towards highly soluble and narrow band-gap organic optoelectronic materials
Largely π-extended naphthalenediimides were synthesized by using efficient palladium catalyzed C–H/C–H homocouplings, with the yields of up to 94%.
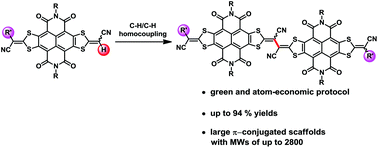
Org. Chem. Front., 2017,4, 823-827
https://doi.org/10.1039/C7QO00061H
Anthroxyl-based biradical: toward the construction of highly stable multi-spin systems
A new two-spin system having two anthroxyl radicals was found to be stable even after exposing it to refluxing ethanol.
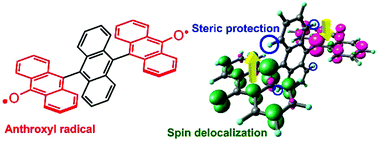
Org. Chem. Front., 2017,4, 828-833
https://doi.org/10.1039/C7QO00130D
Functionalization of pentacene-5,7,12,14-tetraone with geminal enediyne and 1,3-dithiole groups
Pentacene-5,7,12,14-tetraone was subjected to selective olefination and cross-coupling reactions to yield a new class of pentacene-based π-conjugated systems with intriguing structural, electronic, and redox properties.
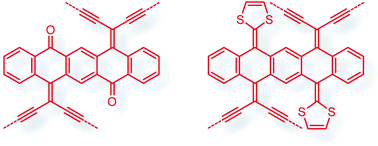
Org. Chem. Front., 2017,4, 804-810
https://doi.org/10.1039/C7QO00041C
Electronic and steric effects on the three-fold Scholl-type cycloheptatriene ring formation around a tribenzotriquinacene core
Single and triple bay-bridging Scholl-type cyclizations of several 1,4,8-triaryl-substituted tribenzotriquinacenes are subject to pronounced electronic and steric substituent effects.
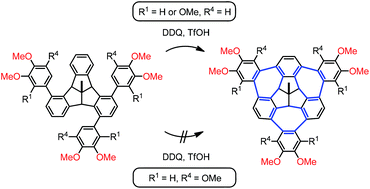
Org. Chem. Front., 2017,4, 817-822
https://doi.org/10.1039/C7QO00132K
Twisted terrylene dyes: synthesis and application in organic solar cells
Two twisted terrylene diimides as non-fullerene acceptors for solar cells have been designed with a resulting power conversion efficiency of 3.64%.
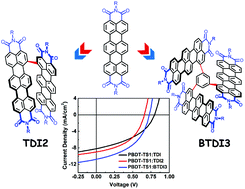
Org. Chem. Front., 2017,4, 811-816
https://doi.org/10.1039/C7QO00118E
The effect of a highly twisted C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond on the electronic structures of 9,9′-bifluorenylidene derivatives in the ground and excited states
C double bond on the electronic structures of 9,9′-bifluorenylidene derivatives in the ground and excited states
A highly twisted C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond elicits changes in the physicochemical properties of π-systems.
C double bond elicits changes in the physicochemical properties of π-systems.
![Graphical abstract: The effect of a highly twisted C [[double bond, length as m-dash]] C double bond on the electronic structures of 9,9′-bifluorenylidene derivatives in the ground and excited states](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C7QO00125H&imageInfo.ImageIdentifier.Year=2017)
Org. Chem. Front., 2017,4, 650-657
https://doi.org/10.1039/C7QO00125H
Tuning liquid crystalline phase behaviour in columnar crown ethers by sulfur substituents
Sulfur-containing side chains in the periphery of o-terphenyl or triphenylene units of crown ethers induce room-temperature columnar mesophases.
Org. Chem. Front., 2017,4, 790-803
https://doi.org/10.1039/C7QO00077D
Poly[2(6)-aminoazulene]: synthesis, photophysical properties, and proton conductivity
Dimeric aminoazulene and poly[2(6)-aminoazulene] are synthesized by Buchwald–Hartwig coupling of the corresponding monomeric carbamatoaminozulenes followed by hydrolysis.
![Graphical abstract: Poly[2(6)-aminoazulene]: synthesis, photophysical properties, and proton conductivity](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C7QO00087A&imageInfo.ImageIdentifier.Year=2017)
Org. Chem. Front., 2017,4, 773-778
https://doi.org/10.1039/C7QO00087A
A theoretical study on quasi-one-dimensional open-shell singlet ladder oligomers: multi-radical nature, aromaticity and second hyperpolarizability
Quasi-one-dimensional open-shell singlet ladder oligomers composed of indenofluorene-like units show strong correlation between multiradical nature, aromaticity and second hyperpolarizability.
Org. Chem. Front., 2017,4, 779-789
https://doi.org/10.1039/C7QO00108H
meso-to-meso PtII-bridged NiII-porphyrin dimers
The synthesis and characterization of a series of meso-to-meso PtII-bridged NiII-porphyrin dimers are described.
Org. Chem. Front., 2017,4, 767-772
https://doi.org/10.1039/C7QO00093F
A new fluorophore displaying remarkable solvatofluorochromism and solid-state light emission, and serving as a turn-on fluorescent sensor for cyanide ions
A new fluorophore displays remarkable solvatofluorochromism, a CIEE effect, and an intense blue-green fluorescence in the presence of cyanide ions.
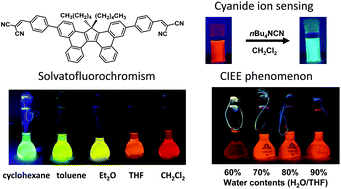
Org. Chem. Front., 2017,4, 743-749
https://doi.org/10.1039/C7QO00029D
Synthesis of homoazafullerene [C59N(CH2)]R and azahomoazafullerene [C59N(NH)]R
The selective insertion of a CH2 or NH group is achieved through the PCl5 induced reaction of a CH2OH or NHOH group, respectively.
![Graphical abstract: Synthesis of homoazafullerene [C59N(CH2)]R and azahomoazafullerene [C59N(NH)]R](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C7QO00098G&imageInfo.ImageIdentifier.Year=2017)
Org. Chem. Front., 2017,4, 750-754
https://doi.org/10.1039/C7QO00098G
Synthesis and properties of electron accepting star-shaped phosphaviologen oligomers
A straightforward synthetic route toward redox-active tri- and tetrastar phosphaviologen oligomers that show the intriguing ability of storing up to 8 electrons is reported.
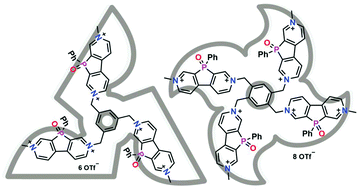
Org. Chem. Front., 2017,4, 717-723
https://doi.org/10.1039/C6QO00872K
The influence of the central acceptor unit on the optoelectronic properties and photovoltaic performance of A–D–A–D–A-type co-oligomers
A series of A–D–A–D–A co-oligomers was developed and implemented as donor materials in solution-processable organic solar cells showing the dependence of molecular structures on the power conversion efficiency upon solvent vapor annealing.
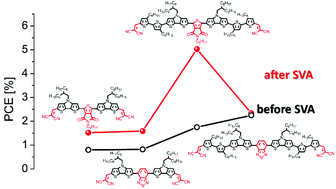
Org. Chem. Front., 2017,4, 755-766
https://doi.org/10.1039/C7QO00043J
Synthesis, solvent-dependent emission and two-photon absorption of a triangular –[D–π–A]3– macrocycle
A large π–conjugated macrocycle featuring a –[D–π–A]3– backbone is synthesized, exhibiting strongly solvent-dependent fluorescence and evident two-photon absorption ability.
![Graphical abstract: Synthesis, solvent-dependent emission and two-photon absorption of a triangular –[D–π–A]3– macrocycle](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6QO00845C&imageInfo.ImageIdentifier.Year=2017)
Org. Chem. Front., 2017,4, 737-742
https://doi.org/10.1039/C6QO00845C
The impact of interplay between electronic and steric effects on the synthesis and the linear and non-linear optical properties of diketopyrrolopyrrole bearing benzofuran moieties
Benzofuran has been proven to be the versatile substituent for tuning the optics of diketopyrrolopyrroles.
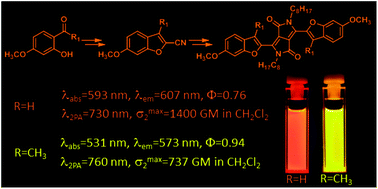
Org. Chem. Front., 2017,4, 724-736
https://doi.org/10.1039/C6QO00869K
Fast construction of dianthraceno[a,e]pentalenes for OPV applications
A new method based on a palladium-catalyzed tandem reaction for the fast construction of a dianthraceno[a,e]pentalene (DAP) framework is developed, which gave a series of dianthraceno[a,e]pentalenes with good functional group tolerance, and it should be highlighted that five C–C bonds were formed in one reaction.
![Graphical abstract: Fast construction of dianthraceno[a,e]pentalenes for OPV applications](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6QO00867D&imageInfo.ImageIdentifier.Year=2017)
Org. Chem. Front., 2017,4, 711-716
https://doi.org/10.1039/C6QO00867D
5-Azadibenzo[a,g]corannulene
The first azacorannulene with a nitrogen on the rim, has been synthesized in seven steps from 4-bromoisoquinoline.
![Graphical abstract: 5-Azadibenzo[a,g]corannulene](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6QO00831C&imageInfo.ImageIdentifier.Year=2017)
Org. Chem. Front., 2017,4, 688-698
https://doi.org/10.1039/C6QO00831C
Selective thionation of naphtho[2,3-b]thiophene diimide: tuning of the optoelectronic properties and packing structure
Naphtho[2,3-b]thiophene diimide was selectively thionated to afford the corresponding dicarboxy-dithiocarboxy diimide with a low-lying LUMO of −4.2 eV.
![Graphical abstract: Selective thionation of naphtho[2,3-b]thiophene diimide: tuning of the optoelectronic properties and packing structure](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6QO00871B&imageInfo.ImageIdentifier.Year=2017)
Org. Chem. Front., 2017,4, 704-710
https://doi.org/10.1039/C6QO00871B
From tetrabenzoheptafulvalene to sp2 carbon nano-rings
A new kind of sp2 carbon nano-rings containing a heptagon and an octagon was synthesized by taking advantage of the “C” shape of syn-tetrabenzoheptafulvalene.
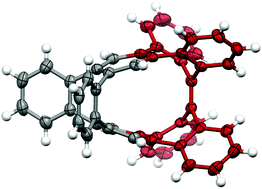
Org. Chem. Front., 2017,4, 699-703
https://doi.org/10.1039/C6QO00828C
Quasi-planar diazadithio and diazodiseleno[8]circulenes: synthesis, structures and properties
Diazadithio and diazadiseleno[8]circulenes were synthesized in good yields through a non-pyrolytic cyclization starting from nitrogen-bridged tetraphenylene.
![Graphical abstract: Quasi-planar diazadithio and diazodiseleno[8]circulenes: synthesis, structures and properties](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6QO00662K&imageInfo.ImageIdentifier.Year=2017)
Org. Chem. Front., 2017,4, 682-687
https://doi.org/10.1039/C6QO00662K
The synthesis, structure, and properties of 5,6,11,12-tetraarylindeno[1,2-b]fluorenes and their applications as donors for organic photovoltaic devices
Three new highly twisted 5,6,11,12-tetraarylindeno[1,2-b]fluorenes have been synthesized, characterized with X-ray analysis, and utilized as electron donors in organic photovoltaics.
![Graphical abstract: The synthesis, structure, and properties of 5,6,11,12-tetraarylindeno[1,2-b]fluorenes and their applications as donors for organic photovoltaic devices](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6QO00673F&imageInfo.ImageIdentifier.Year=2017)
Org. Chem. Front., 2017,4, 675-681
https://doi.org/10.1039/C6QO00673F
Polymerization of acetylene: polyynes, but not carbyne
Polymerization of acetylene in the presence of sterically-hindered endgroups leads to polyynes, but with lengths shorter than by stepwise syntheses.
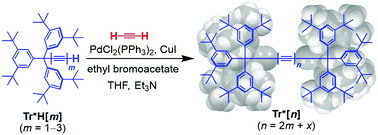
Org. Chem. Front., 2017,4, 668-674
https://doi.org/10.1039/C6QO00648E
Synthesis of a figure-eight azahelicene dimer with high emission and CPL properties
A bisbutadiyne bridged azahelicene dimer with a figure-eight shape, which exhibited both high fluorescence quantum yield and CPL properties, has been synthesized through the Glaser–Hay homo-coupling reaction with diethynylazahelicene.
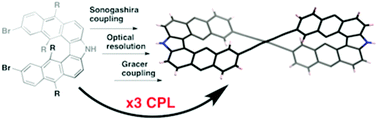
Org. Chem. Front., 2017,4, 664-667
https://doi.org/10.1039/C6QO00629A
Donor- and acceptor-functionalized dibenzo[a,e]pentalenes: modulation of the electronic band gap
The syntheses, and optoelectronic and structural properties of 2,7-donor- and acceptor-functionalized dibenzo[a,e]pentalenes, accessed in a versatile synthetic route, are presented and discussed.
![Graphical abstract: Donor- and acceptor-functionalized dibenzo[a,e]pentalenes: modulation of the electronic band gap](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6QO00487C&imageInfo.ImageIdentifier.Year=2017)
Org. Chem. Front., 2017,4, 658-663
https://doi.org/10.1039/C6QO00487C
Synthesis of a quinoidal dithieno[2,3-d;2′,3′-d]benzo[2,1-b;3,4-b′]-dithiophene based open-shell singlet biradicaloid
A fused heteroacene derivative, bis(dicyanomethylene)-end-capped-dithieno[2,3-d;2′,3′-d]benzo[2,1-b;3,4-b′]-dithiophene (4CN-DTmBDT) was synthesized.
![Graphical abstract: Synthesis of a quinoidal dithieno[2,3-d;2′,3′-d]benzo[2,1-b;3,4-b′]-dithiophene based open-shell singlet biradicaloid](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6QO00543H&imageInfo.ImageIdentifier.Year=2017)
Org. Chem. Front., 2017,4, 18-21
https://doi.org/10.1039/C6QO00543H
A cross-coupling-annulation cascade from peri-dibromonaphthalimide to pseudo-rylene bisimides
A novel synthetic strategy based on cascade C–C cross-coupling and C–H arylation afforded structurally unique polycyclic aromatics with desirable optical and redox properties.
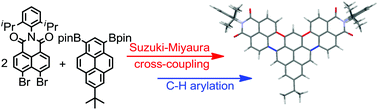
Org. Chem. Front., 2016,3, 1435-1442
https://doi.org/10.1039/C6QO00421K
About this collection
Novel pi-electron molecular scaffolds enable clarification of fundamental questions of chemical bonding and can also operate as functional materials for diverse applications such as fluorescence sensing, photoswitching, or organic electronics.
This themed collection aims to showcase the very recent efforts in designing exciting new structures, developing unprecedented synthetic methods, illustrating structure-property relationships, and also the investigations into (anti-)aromaticity, optical or redox properties, or conformational features of pi-electron systems.
Guest Editors of this themed collection are Professor Frank Würthner (University of Würzburg, Germany), Professor Michael Haley (University of Oregon, USA) and Professor Nazario Martín (University Complutense Madrid, Spain).