Polycyclic heteroaromatic hydrocarbons containing a benzoisoindole core†
Abstract
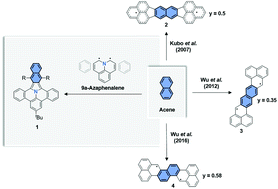
By the combination of 9a-azaphenalene and a perpendicularly oriented acene, we have synthesized three derivatives of a series of novel, fully-conjugated nitrogen-containing polycyclic aromatic hydrocarbons (PAHs), namely [7,8]naphtho[2′,3′:1,2]indolizino[6,5,4,3-def]phenanthridine, with an acetylene triisopropylsilyl (TIPS), phenyl or benzothiophenyl substituent. Their optoelectronic properties were studied via UV-Vis-NIR absorption, fluorescence spectroscopy and cyclic voltammetry. In addition, in situ spectroelectrochemistry was performed to investigate the optical and magnetic properties of the mono-radical cation and anion by quasi-reversible oxidation and reduction of 11-(tert-butyl)-5,17-bis((triisopropylsilyl)ethynyl)[7,8]naphtho[2′,3′:1,2]indolizino[6,5,4,3-def]phenanthridine (1a). Theoretical modelling confirmed the predominately closed-shell electronic ground state with a weak diradical character depending on the geometry.
- This article is part of the themed collection: Novel π-electron molecular scaffolds


 Please wait while we load your content...
Please wait while we load your content...