Themed collection 2023 Organic Chemistry Frontiers HOT articles

Ligand relay catalysis: a newly emerged synthetic strategy
Ligand relay catalysis, which brings a new way to broaden the SMMLC strategy, has recently been developed and discussed. This strategy acts as a powerful way, broadening the range of catalytic transformations to construct highly functional molecules.

Org. Chem. Front., 2023,10, 4146-4160
https://doi.org/10.1039/D3QO00913K
Directing group-assisted selective C–H activation of six-membered N-heterocycles and benzo-fused N-heterocycles
Directing group-assisted selective C–H bond activation of six-membered N-heterocycles and benzo-fused N-heterocycles has been reported.

Org. Chem. Front., 2024,11, 540-575
https://doi.org/10.1039/D3QO01396K
A route to carbon-sp3 bridging spiro-molecules: synthetic methods and optoelectronic applications
Molecules with spiro-linked π-conjugated structures have attracted considerable attention in the realm of organic functional materials due to their advantageous structural features.
Org. Chem. Front., 2024,11, 508-539
https://doi.org/10.1039/D3QO01735D
Tetraphenylethene-based macrocycles with dual-ring topology: synthesis, structures, and applications
This review focuses on the eight-shaped double ring structure of tetraphenylethylene, summarizes the research progress of structures, ionic molecular structures and metal molecular structures, and looks forward to its future development direction.

Org. Chem. Front., 2023,10, 6225-6239
https://doi.org/10.1039/D3QO01426F
BX3-mediated borylation for the synthesis of organoboron compounds
This review summarizes the recent progress in borylation driven by BX3 in the fields of organic synthesis and drug synthesis.

Org. Chem. Front., 2023,10, 6010-6020
https://doi.org/10.1039/D3QO01487H
Nucleophilic organocatalysis involving radical intermediates
The recent advancements in single-electron nucleophilic organocatalysis that facilitates single-electron transfer between substrates through the formation of a covalent intermediate were summarized based on the type of catalyst.

Org. Chem. Front., 2023,10, 6021-6040
https://doi.org/10.1039/D3QO01435E
Recent advances in trifluoroethylation reaction
The recent advances in trifluoroethylation reactions were summarized based on the bond formation: C(sp)–CH2CF3, C(sp2)–CH2CF3, C(sp3)–CH2CF3, and X (X = N, O, S, Se)–CH2CF3.

Org. Chem. Front., 2023,10, 5986-6009
https://doi.org/10.1039/D3QO01366A
Advancement of vinylene carbonate as a coupling partner in metal-catalyzed C–H functionalization
Vinylene carbonate (VC) has emerged as a promising coupling partner to participate in various attractive C–H conversions and implement an increasing number of novel reactions. In this review, we provide a summary of the advancements achieved in metal-catalyzed C–H functionalization using VC.
Org. Chem. Front., 2023,10, 5717-5734
https://doi.org/10.1039/D3QO01227A
Recent advances in three-component radical acylative difunctionalization of unsaturated carbon–carbon bonds
This review highlights the recent advances in radical acylated difunctionalization of unsaturated carbon–carbon bonds and focuses on the mechanistic insights of these transformations.

Org. Chem. Front., 2023,10, 4488-4515
https://doi.org/10.1039/D3QO01010D
Lighting the way to diverse cyclic architectures: expanding the horizons with photogenerated ketenes in sustainable chemistry
Photogenerated ketenes simplify and accelerate the synthesis of diverse cyclic architectures and demonstrate their promising applications in flow chemistry.

Org. Chem. Front., 2023,10, 4474-4487
https://doi.org/10.1039/D3QO00831B
Catalyst-controlled stereoselective carbon–heteroatom bond formations by N-heterocyclic carbene (NHC) organocatalysis
This review highlights recent advances in stereoselective carbon–heteroatom bond forming reactions, including C–N, C–O, C–S, C–F, C–P, etc., that were enabled by NHC organocatalysis with a focus on new activation modes and reactive intermediates.

Org. Chem. Front., 2023,10, 4437-4458
https://doi.org/10.1039/D3QO00667K
Recent advances in the photocatalytic synthesis of aldehydes
This review summarizes the recently developed photocatalytic strategies for the installation of the formyl group into various scaffolds.

Org. Chem. Front., 2023,10, 4198-4210
https://doi.org/10.1039/D3QO01026K
New advances in chiral nanographene chemistry
This review will highlight several recent and lesser-reviewed works related to the preparation of chiral nanographenes, specifically focusing on structures prepared by non-Scholl methods.
Org. Chem. Front., 2023,10, 4167-4197
https://doi.org/10.1039/D3QO00517H
Recent advances in electrochemical synthesis of diversely functionalized oxindole derivatives
This review will focus on the electrochemical synthesis of diversified functionalized oxindole derivatives through C-S, C-O, C-C, C-Se, and C-N bond formation over the past decade.

Org. Chem. Front., 2023,10, 3929-3950
https://doi.org/10.1039/D3QO00671A
Transition-metal-catalyzed asymmetric defluorinative reactions
The latest achievements in transition-metal-catalyzed enantioselective defluorinative coupling reactions have been comprehensively summarized on the basis of the classification of transition-metal catalysts.

Org. Chem. Front., 2023,10, 3909-3928
https://doi.org/10.1039/D3QO00751K
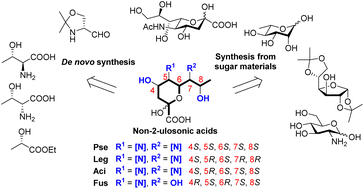
Recent progress on the chemical synthesis of bacterial non-2-ulosonic acids
A summary of the chemical syntheses of bacterial non-2-ulosonic acids and their derivatives via de novo synthetic approaches starting from non-sugar compounds and total synthesis starting from sugar material.
Org. Chem. Front., 2023,10, 3429-3446
https://doi.org/10.1039/D3QO00637A
Recent advances in intramolecular kinetic resolution reactions
This review summarizes the advances in the field of intramolecular kinetic resolution (KR) mediated by non-enzymatic catalysts. The relative classification of intramolecular KR is accomplished and several categories of reactions are discussed.

Org. Chem. Front., 2023,10, 3401-3428
https://doi.org/10.1039/D3QO00563A
Applications of trisulfide radical anion S3˙− in organic synthesis
The synthetic and mechanistic aspects of the role of the trisulfide radical anion S3˙− in organic chemistry are reviewed.

Org. Chem. Front., 2023,10, 3378-3400
https://doi.org/10.1039/D3QO00496A
Electrochemical synthesis and transformation of organoboron compounds
This review highlights the recent advances in both electrochemical borylation and hydroboration to synthesize organoboron compounds and electrochemical transformation of organoboron compounds to construct carbon–carbon and carbon–heteroatom bonds.
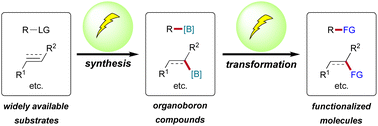
Org. Chem. Front., 2023,10, 3361-3377
https://doi.org/10.1039/D3QO00486D
Recent advances in the asymmetric catalytic construction of oxa-quaternary carbon centers
This review focuses on the very recent advances (from 2020 to the beginning of 2023) in enantioselective catalytic reactions for the construction of oxa-quaternary stereocenters.
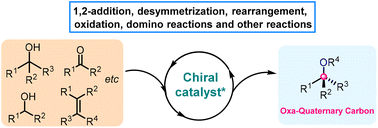
Org. Chem. Front., 2023,10, 3110-3129
https://doi.org/10.1039/D3QO00527E
Progress in catalytic asymmetric α-functionalization of vinylogous nucleophilic species
This review summarizes a view of the advances in the catalytic asymmetric α-functionalization of vinylogous nucleophilic species, the content of which is categorized based on the type of vinylogous donor.
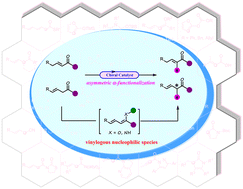
Org. Chem. Front., 2023,10, 3130-3168
https://doi.org/10.1039/D3QO00506B
Recent advances in the electrochemical generation of 1,3-dicarbonyl radicals from C–H bonds
Recent advances in the electrochemical generation of 1,3-dicarbonyl radicals from C–H bonds and their mechanistic insights and synthetic applications have been summarized.
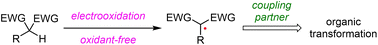
Org. Chem. Front., 2023,10, 2830-2848
https://doi.org/10.1039/D3QO00408B
Recent advances in the cyclization reactions of pyridinium 1,n-zwitterions (n = 4 and 5): scope and mechanism
This review summarizes the recent advances in cyclization reactions involving pyridinium 1,n-zwitterions (n = 4 and 5) and highlights the applications of pyridinium 1,n-zwitterions (n = 4 and 5) in the efficient construction of heterocycles.
Org. Chem. Front., 2023,10, 2813-2829
https://doi.org/10.1039/D3QO00228D
Photoredox chromium and cobalt dual catalysis for carbonyl allylation with butadiene via allyl radical intermediates
The allylation of aldehydes with dienes was achieved using cobalt and chromium catalysts through a metal-hydride hydrogen atom transfer (MHAT) pathway. The novel reaction resulted in the formation of various homo-allyl alcohols with excellent diastereoselectivity.

Org. Chem. Front., 2024,11, 684-689
https://doi.org/10.1039/D3QO01403G
Asymmetric total synthesis of montanine-type amaryllidaceae alkaloids
The first asymmetric total syntheses of (+)-pancratinines B and C and total syntheses of (−)-montanine, (−)-pancracine and (−)-brunsvigine were realized in a divergent fashion. Our synthetic strategy features a copper-catalyzed [3 + 2] annulation reaction and a Pictet–Spengler cyclization.

Org. Chem. Front., 2024,11, 668-672
https://doi.org/10.1039/D3QO01835K
N-heterocyclic nitrenium charge transfer catalysis via inner-sphere electron transfer in concert with halogen-atom dissociation
We report an efficient and versatile catalytic N-heterocyclic nitrenium charge transfer complex strategy for the single-electron reduction of chloroform, resulting in the generation of the dichloromethyl radical for use in various transformations.

Org. Chem. Front., 2024,11, 673-683
https://doi.org/10.1039/D3QO01779F
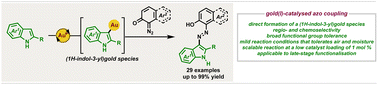
Gold catalysed regio- and chemoselective azo coupling of 1,2- and 1,4-diazoquinones with 1H-indoles
A synthetic method that enables the regio- and chemoselective gold(I)-catalysed azo coupling of 1,2- and 1,4-diazoquinones with 1H-indoles to give a wide range of (E)-3-arylazoindoles is reported.
Org. Chem. Front., 2024,11, 290-298
https://doi.org/10.1039/D3QO01676E
Isoxerophilins A and B, two diterpene heterodimers from Isodon xerophilus: structural elucidation and semisynthesis of isoxerophilin analogues
Two unprecedented diterpene heterodimers, isoxerophilins A and B (1 and 2), were isolated from Isodon xerophilus. A bioinspired semisynthesis of ten isoxerophilin analogues was accomplished. Compound 12 exhibited obvious latent tumor immunotherapy activity.

Org. Chem. Front., 2024,11, 37-46
https://doi.org/10.1039/D3QO01679J
Copper-catalyzed 1,2,2-trifunctionalization of maleimides with 1,7-enynes and oxime esters via radical relay/1,5-hydrogen-atom transfer
We describe a novel 1,2,2-trifunctionalization of maleimides with 1,7-enynes and oxime esters through simultaneous construction of four C−C bonds and a C≡N bond in one pot based on the radical relay/1,5-hydrogen-atom transfer (HAT).

Org. Chem. Front., 2023,10, 6096-6102
https://doi.org/10.1039/D3QO01431B
Twisted organic TADF triads based on a diindolocarbazole donor for efficient photoisomerization of stilbene and photo-arylation of heteroarenes
Twisted D–A TADF triads DI-PF and DI-PI based on a diindolocarbazole donor and dibenzo[a,c]phenazine (PF) and phenanthro[9,10-d]imidazole (PI) acceptors were utilized for E–Z photoisomerization of stilbene and C–H arylation of heteroarenes.

Org. Chem. Front., 2023,10, 6087-6095
https://doi.org/10.1039/D3QO01542D
Enantioselective construction of quaternary stereocenters via organocatalytic arylation of isoxazolin-5-ones with o-quinone diimides
A bifunctional squaramide catalyzes the enantioselective arylation of isoxazolin-5-ones with o-quinone diimides (o-QDIs) to give isoxazolin-5-ones featuring an arylated quaternary stereocenter in high yields and excellent enantioselectivities.

Org. Chem. Front., 2023,10, 6081-6086
https://doi.org/10.1039/D3QO01600E
Highly diastereo- and enantioselective synthesis of spiro β-lactams via copper-catalyzed Kinugasa/aldol cascade reaction
A copper(I)-catalyzed highly diastereo- and enantioselective Kinugasa/aldol cascade reaction of N-(2-acylaryl) propiolamides with nitrones has been demonstrated for efficient synthesis of structurally novel chiral spiro β-lactams.

Org. Chem. Front., 2023,10, 5383-5388
https://doi.org/10.1039/D3QO01362F
Direct C(sp2)–H fluoroalkylation of quinoxalin-2(1H)-ones with (fluoroalkyl)triphenylphosphonium salts and alkenes
A photoredox-catalyzed direct C(sp2)–H fluoroalkylation of quinoxalin-2(1H)-ones with (fluoroalkyl)triphenylphosphonium salts and alkenes is described, providing a practical approach for the generation of diverse fluoroalkyl-containing quinoxalin-2(1H)-ones.

Org. Chem. Front., 2023,10, 5375-5382
https://doi.org/10.1039/D3QO01247F
Electrocatalytic oxidative C–H cycloamination towards tricyclic [1,2,4]triazolo-[3,4-i]purine nucleosides mediated by bromide ions
A green and sustainable electrochemical alternative strategy for the synthesis of biologically important [1,2,4]-triazolo-[3,4-i]-purine derivatives via intramolecular oxidative C–N bond coupling has been established.
![Graphical abstract: Electrocatalytic oxidative C–H cycloamination towards tricyclic [1,2,4]triazolo-[3,4-i]purine nucleosides mediated by bromide ions](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D3QO01050C&imageInfo.ImageIdentifier.Year=2023)
Org. Chem. Front., 2023,10, 5369-5374
https://doi.org/10.1039/D3QO01050C
Electrochemical multicomponent reaction toward vicinal sulfenyltetrazolation of unactivated alkenes
An electrochemical multicomponent reaction (e-MCR) enables the green and sustainable preparation of diverse vicinal sulfenyltetrazolation using the readily available unactivated olefins, thiols, azidotrimethylsilane, and acetonitrile.

Org. Chem. Front., 2023,10, 5064-5069
https://doi.org/10.1039/D3QO01241G
Phosphine-catalyzed asymmetric aza-Morita–Baylis–Hillman reaction of endocyclic ketimines and activated alkenes
Asymmetric aza-Morita–Baylis–Hillman reaction involving endocyclic ketimines, tetra-substituted chiral carbon center was achieved.

Org. Chem. Front., 2023,10, 5076-5082
https://doi.org/10.1039/D3QO01047C
Palladium-catalyzed highly chemoselective dearomative spirocyclization of Ugi adducts: facile access to functionalized benzoazepinespiroindolenines with diastereoselectivity
The development of chemoselective strategies for complex molecules is intrinsically challenging. In this paper, a facile and diversity-oriented access to benzoazepinespiroindolenines is elaborated via Pd-catalyzed highly chemoselective dearomative spirocyclization of Ugi adducts.

Org. Chem. Front., 2023,10, 5083-5091
https://doi.org/10.1039/D3QO01262J
Asymmetric hydrogenation of all-carbon tetrasubstituted α-acylpyrazole-β-alkyl cycloalkenes
A general method for Ir-catalyzed asymmetric hydrogenation of tetrasubstituted α-acylpyrazole-β-alkyl cycloalkenes has been developed, furnishing 1,2-cis substituted carbo- or heterocycles with high yields and excellent enantioselectivities.

Org. Chem. Front., 2023,10, 5070-5075
https://doi.org/10.1039/D3QO01196H
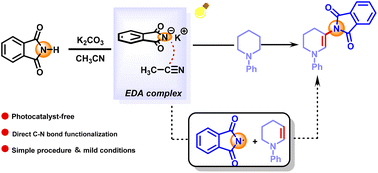
Photoinduced desaturative β-C(sp3)–H amidation of N-phenylpiperidine with phthalimide driven by electron donor–acceptor complexes
A photocatalytic method for desaturative C(sp3)–H amidation of N-phenylpiperidine under external photocatalyst- and transition metal-free conditions.
Org. Chem. Front., 2023,10, 4758-4763
https://doi.org/10.1039/D3QO01030A
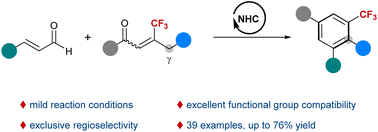
N-Heterocyclic carbene catalyzed synthesis of benzotrifluorides from enals and β-trifluoromethylenones
An NHC-catalyzed benzannulation reaction of enals and β-trifluoromethylenones is developed for the synthesis of benzotrifluorides.
Org. Chem. Front., 2023,10, 4774-4778
https://doi.org/10.1039/D3QO01222K
An iron-catalyzed C–S bond-forming reaction of carboxylic acids and hydrocarbons via photo-induced ligand to metal charge transfer
A photo-induced iron-catalyzed strategy that facilitates the transformation of carboxylic acids and hydrocarbons into thioethers and sulfoxides is herein reported.

Org. Chem. Front., 2023,10, 4764-4773
https://doi.org/10.1039/D3QO01081C
Sodiated Oppolzer enolates: solution structures, mechanism of alkylation, and origin of stereoselectivity
Sodium camphorsultam enolates are structurally characterized uncovering monomers and dimers; rate studies reveal ionic transition structures and curious solvation effects.

Org. Chem. Front., 2023,10, 4750-4757
https://doi.org/10.1039/D3QO01021J
Visible-light-induced defluorinative allylation of difluoromethyl ketones using alkylamines as bifunctional agents
A visible-light-induced alkylamine-facilitated reductive defluoroallylation of difluoromethyl ketones with allylic chlorides is reported, enabling efficient access to α-fluoro alkenylketones with high efficiency.

Org. Chem. Front., 2023,10, 4542-4549
https://doi.org/10.1039/D3QO00870C
Unveiling the mechanism and origin of stereocontrol in dinuclear-zinc-catalyzed reductive desymmetrization of malonic esters
Mechanism of catalytic desymmetrization of malonic esters have been explored, which provides molecular-level insights on how the ligand-casted chiral scaffolding can be intentionally applied to differentiate symmetrical elements in desymmetrization.

Org. Chem. Front., 2023,10, 4529-4541
https://doi.org/10.1039/D3QO00846K
Photocatalytic C(sp2)–OMe bond cleavage and amidation of anisoles with N-hydroxyphthalimides and phthalimides
The cleavage of the inert C(sp2)-OMe bond was developed under a metal-free photocatalytic strategy using 4CzIPN as a mild oxidizing photoredox catalyst.

Org. Chem. Front., 2023,10, 4522-4528
https://doi.org/10.1039/D3QO00861D
Supramolecular protection with a recyclable molecular container: an efficient strategy for the one-pot selective functionalization of polyfunctional substrates
The use of a supramolecular protecting molecular container, whose host-guest properties are under acid–base control, enables the functionalization with high chemo-, regio- and iteroselectivities of polyamines in a one-pot cyclic process.
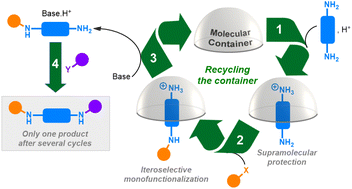
Org. Chem. Front., 2023,10, 4230-4242
https://doi.org/10.1039/D3QO00804E
Redox transformation of β-sulfinyl esters for asymmetric synthesis of sulfone-based axially chiral styrenes
An unprecedented catalytic sulfonylation strategy has been developed through the redox transformation of β-sulfinyl esters, providing a facile access to axially chiral sulfone-containing styrenes with VQMs in excellent enantioselectivities.

Org. Chem. Front., 2023,10, 3975-3981
https://doi.org/10.1039/D3QO00932G
The role of attractive dispersion interaction in promoting the catalytic activity of asymmetric hydrogenation
This study proves Wanbin Zhang's finding that weak attractive interactions between substrate and ligand play an important role in metal-catalyzed asymmetric hydrogenations and shows that the dispersion interaction is the major attractive interaction.

Org. Chem. Front., 2023,10, 3485-3490
https://doi.org/10.1039/D3QO00332A
Photo-induced cyclization of olefinic amides towards sulfonamidylated iminoisobenzofurans and benzoxazines
A visible-light induced radical cyclization of olefinic amides with easily available N-sulfonylaminopyridinium salts towards iminoisobenzofurans and benzoxazines has been developed.

Org. Chem. Front., 2023,10, 3479-3484
https://doi.org/10.1039/D3QO00675A
Transition metal-free and regioselective alkenyl C–S cross-coupling reaction of alkenylsulfonium salts
A novel and highly efficient strategy for the construction of diverse alkenyl sulfoxides via transition metal-free C–S cross-coupling of sulfenate anions with alkenylsulfonium salts is reported.

Org. Chem. Front., 2023,10, 2907-2912
https://doi.org/10.1039/D3QO00507K
Artemeriopolides A–D, two types of sesquiterpenoid dimers with rare carbon skeletons from Artemisia eriopoda and their antihepatoma cytotoxicity
Artemeriopolides A–D (1–4), four novel cadinane sesquiterpenoid dimers featuring the rare 2-oxaspiro[4.6]undecan-1,7-dione, 2-oxaspiro[4.5]decan-1-one, and 2-oxaspiro[5.5]undecan-1-one ring systems, were isolated from Artemisia eriopoda.
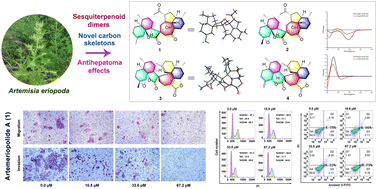
Org. Chem. Front., 2023,10, 2635-2641
https://doi.org/10.1039/D3QO00223C
DFT study on stereoselective Rh-catalyzed intramolecular [2 + 2 + 2] cycloaddition of allene–ene–ynes
CAT catalyzes the reaction of 1a to generate intermediate INT2, which is a common intermediate to generate final products 2a and 3a.
![Graphical abstract: DFT study on stereoselective Rh-catalyzed intramolecular [2 + 2 + 2] cycloaddition of allene–ene–ynes](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D3QO00363A&imageInfo.ImageIdentifier.Year=2023)
Org. Chem. Front., 2023,10, 2624-2634
https://doi.org/10.1039/D3QO00363A
2,7-Dinitrophenanthrene-9,10-dione as a photosensitizer for the dehydrogenative lactonization of 2-arylbenzoic acids
This report describes the visible-light-induced C–H/COO–H oxidative coupling reaction of 2-arylbenzoic acids to synthesize benzo-3,4-coumarins by using 2,7-dinitrophenanthrene-9,10-dione as a photocatalyst.
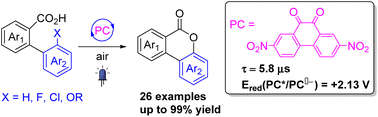
Org. Chem. Front., 2023,10, 2429-2433
https://doi.org/10.1039/D3QO00368J
Catalytic asymmetric [4 + 1] cycloaddition to synthesize chiral pyrazoline-spirooxindoles
The first asymmetric [4 + 1] annulation of oxindole 3-pyridinium salts and hydrazones is realized by using a chiral N,N′-dioxide-Pr(OTf)3 complex. A range of bioactive chiral pyrazoline-spirooxindoles are obtained in good yields with excellent ee values.
![Graphical abstract: Catalytic asymmetric [4 + 1] cycloaddition to synthesize chiral pyrazoline-spirooxindoles](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D3QO00320E&imageInfo.ImageIdentifier.Year=2023)
Org. Chem. Front., 2023,10, 2422-2428
https://doi.org/10.1039/D3QO00320E
Regio- and stereoselective oxidative conversion of alkynes to sulfenylated α,β-unsaturated carbonyls
An electrophilic sulfenylation promoted oxidation of alkynes is presented, providing the chemo-, regio- and stereoselective synthesis of α-sulfenylated α,β-unsaturated aldehydes.
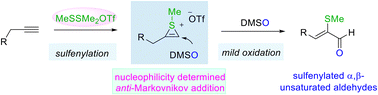
Org. Chem. Front., 2023,10, 2416-2421
https://doi.org/10.1039/D3QO00236E
Visible light mediated regioselective 1,3-oxylallylation of aryl cyclopropanes under redox-neutral conditions
A photoredox catalysed 1,3-oxylallylation of aryl cyclopropanes was accomplished by reaction with carboxylic acids and allyl sulfones.
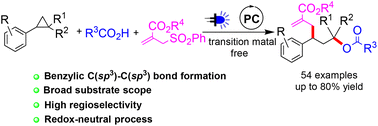
Org. Chem. Front., 2023,10, 2147-2154
https://doi.org/10.1039/D3QO00281K
Chiral oxalamide phosphine (COAP)-Pd-catalyzed enantioselective cascade formal [4 + 1] annulation for enantioenriched 2,3-disubstituted indolines and further DFT study on regio- and stereocontrol
COAP-Pd-catalyzed asymmetric cascade formal [4 + 1] annulation was developed between racemic vinyl benzoxazinones and N-tosylhydrazone sodium salts, affording trans-2,3-disubstituted indolines in good yields with high stereoselectivity.
![Graphical abstract: Chiral oxalamide phosphine (COAP)-Pd-catalyzed enantioselective cascade formal [4 + 1] annulation for enantioenriched 2,3-disubstituted indolines and further DFT study on regio- and stereocontrol](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D3QO00011G&imageInfo.ImageIdentifier.Year=2023)
Org. Chem. Front., 2023,10, 2138-2146
https://doi.org/10.1039/D3QO00011G
Carboxylic acid-assisted sterically demanding reductive cross-coupling between cycloalkenyl and alkyl bromides
Carboxylic acid-assisted sterically demanding reductive cross-coupling between cycloalkenyl and alkyl bromides affords all-carbon tetrasubstituted cycloalkenes under mild reaction conditions in good yields.
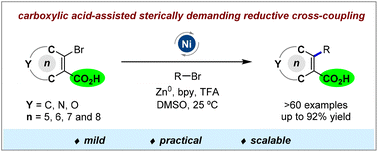
Org. Chem. Front., 2023,10, 1897-1902
https://doi.org/10.1039/D3QO00155E
Copper(I)-catalyzed multicomponent interrupted click reaction: modular synthesis of triazole sulfides from elemental sulfur
A new multicomponent copper(I)-catalyzed interrupted click reaction which allows the rapid modular synthesis of multisubstituted triazole sulfides including various medium-sized and macrocycles in one-step from elemental sulfur was reported.
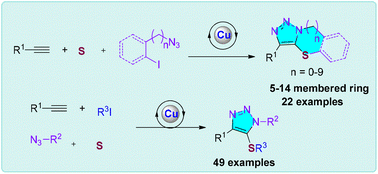
Org. Chem. Front., 2023,10, 1890-1896
https://doi.org/10.1039/D3QO00224A
Photocatalytic dehydrations for the Ritter reaction
A photocatalytic dehydration method was developed to efficiently convert benzyl alcohols to the corresponding amides.

Org. Chem. Front., 2023,10, 1375-1379
https://doi.org/10.1039/D2QO02046G
Construction of methylene-bridged electron-deficient corona[n]arenes and selective fluoride recognition through anion–π interactions
Despite significant development of the nascent anion–π interactions in recent years, the noncovalent interactions between fluoride ion, a unique anion species, and electron-neutral aromatic rings remain rare and even controversial.
![Graphical abstract: Construction of methylene-bridged electron-deficient corona[n]arenes and selective fluoride recognition through anion–π interactions](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D3QO00024A&imageInfo.ImageIdentifier.Year=2023)
Org. Chem. Front., 2023,10, 1405-1411
https://doi.org/10.1039/D3QO00024A
Bis(pentafluorophenyl)borane-catalyzed E-selective isomerization of terminal alkenes to internal alkenes
The bis(pentafluorophenyl)borane-catalyzed E-selective isomerization of terminal alkenes to internal alkenes via a hydroboration/retro-hydroboration sequence is reported.
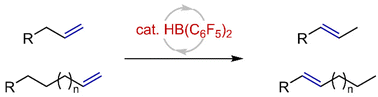
Org. Chem. Front., 2023,10, 1128-1133
https://doi.org/10.1039/D2QO01998A
HTE and machine learning-assisted development of iridium(I)-catalyzed selective O–H bond insertion reactions toward carboxymethyl ketones
By combining HTE and machine learning technologies, an iridium(I)-catalyzed highly selective O–H bond insertion reaction of carboxylic acids and sulfoxonium ylides was developed, and an extensive reaction space exploration was accomplished.
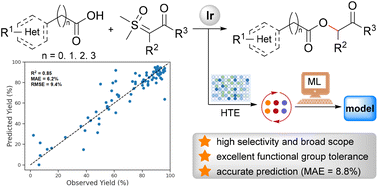
Org. Chem. Front., 2023,10, 1153-1159
https://doi.org/10.1039/D2QO01954J
Photoredox-catalyzed sequential Dowd–Beckwith ring expansion and C–H functionalization of THIQs
An efficient tandem Dowd–Beckwith ring expansion/C–H functionalization of tetrahydroisoquinoline (THIQ) is disclosed upon photocatalysis, which provided a unique thought to connect important medium-sized cycloalkanones with THIQs skeletons together.

Org. Chem. Front., 2023,10, 1147-1152
https://doi.org/10.1039/D2QO02001G
Mechanistic study of nickel-catalyzed intramolecular [4 + 2] cycloaddition of cyclobutanone with allene: origin of selectivity and ligand effect
The transition metal catalyzed cycloaddition reaction is one of the most powerful tools for the construction of carbon- and heterocycles. DFT studies are performed to investigate the mechanism of the Ni-catalyzed intramolecular [4 + 2] cycloaddition.
![Graphical abstract: Mechanistic study of nickel-catalyzed intramolecular [4 + 2] cycloaddition of cyclobutanone with allene: origin of selectivity and ligand effect](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D2QO01849G&imageInfo.ImageIdentifier.Year=2023)
Org. Chem. Front., 2023,10, 1134-1146
https://doi.org/10.1039/D2QO01849G
Visible light-mediated NHC and photoredox co-catalyzed 1,2-sulfonylacylation of allenes via acyl and allyl radical cross-coupling
Visible light-mediated NHC and photoredox co-catalyzed radical 1,2-sulfonylacylation of allenes via cross-coupling between an allyl radical and an NHC-stabilized acyl radical.
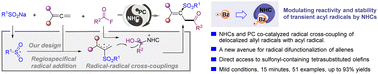
Org. Chem. Front., 2023,10, 1047-1055
https://doi.org/10.1039/D2QO01993K
Photoredox-catalyzed intermolecular azidosulfonylation of alkenes with DABCO·(SO2)2, trimethylsilyl azide and thianthrenium salts
Synthesis of β-azido alkylsulfones through a photoredox-catalyzed azido sulfonylation of alkenes with DABCO·(SO2)2, trimethylsilyl azide and alkyl thianthrenium salts is developed.

Org. Chem. Front., 2023,10, 866-871
https://doi.org/10.1039/D2QO01706G
Photo-induced redox cascade reaction of nitroarenes and amines
A photo-induced redox cascade reaction has been developed for the chemoselective construction of isoxazolidine derivatives from stable and easily available nitroarenes and amines.
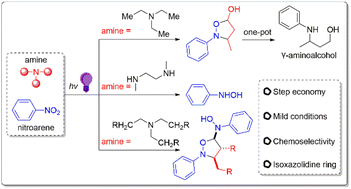
Org. Chem. Front., 2023,10, 624-631
https://doi.org/10.1039/D2QO01743A
Divergent synthesis of quinoxalin-2(1H)-one derivatives through photoinduced C–H functionalization without a photocatalyst
Photo-induced C–H functionalization for divergent synthesis of quinoxalin-2(1H)-one derivatives using H2O2 as an oxidant without a photocatalyst.
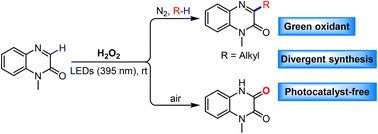
Org. Chem. Front., 2023,10, 611-623
https://doi.org/10.1039/D2QO01531E
In situ generated aminodiborane as a reagent for deoxygenative reduction of carboxamides to amines
The in situ generated aminodiborane (μ-NH2B2H5) using NH3BH3 and elemental iodine (I2) is used for the reduction of carboxamides to amines.
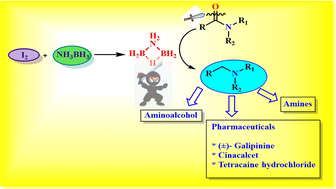
Org. Chem. Front., 2023,10, 327-334
https://doi.org/10.1039/D2QO01717B
About this collection
Read our on-going collection of the hottest research published as advanced article from Organic Chemistry Frontiers in 2023. These articles are recommended by reviewers as being of significant novelty and interest. Congratulations to all the authors whose articles are featured!