Themed collection Photofunctional flavoproteins and UVB photosensors

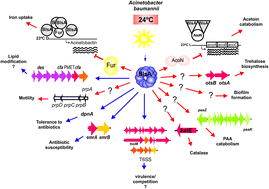
Through the eyes of a pathogen: light perception and signal transduction in Acinetobacter baumannii
Sunlight is a ubiquitous environmental stimulus for the great majority of living organisms on Earth; therefore it is logical to expect the development of “seeing mechanisms” which lead them to successfully adapt to particular ecological niches.
Photochem. Photobiol. Sci., 2019,18, 2363-2373
https://doi.org/10.1039/C9PP00261H
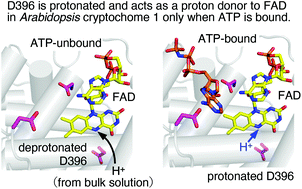
ATP binding promotes light-induced structural changes to the protein moiety of Arabidopsis cryptochrome 1
D396 is protonated and acts as a proton donor to FAD in Arabidopsis cryptochome 1 only when ATP is bound.
Photochem. Photobiol. Sci., 2020,19, 1326-1331
https://doi.org/10.1039/D0PP00003E
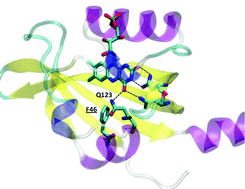
Interplay among the “flipping” glutamine, a conserved phenylalanine, water and hydrogen bonds within a blue-light sensing LOV domain
A combined photoacoustics and molecular dynamics approach highlights the crucial role of a conserved phenyalanine in photosensing LOV domains.
Photochem. Photobiol. Sci., 2020,19, 892-904
https://doi.org/10.1039/D0PP00082E
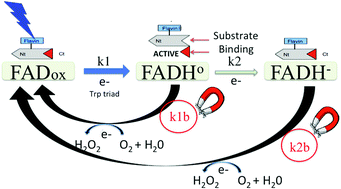
Cryptochrome mediated magnetic sensitivity in Arabidopsis occurs independently of light-induced electron transfer to the flavin
Arabidopsis cryptochrome-dependent magnetosensitivity occurs via a reaction that does not require light. This excludes radical pairs formed during light-triggered electron transfer to the flavin.
Photochem. Photobiol. Sci., 2020,19, 341-352
https://doi.org/10.1039/C9PP00469F
Dimerization of LOV domains of Rhodobacter sphaeroides (RsLOV) studied with FRET and stopped-flow experiments
LOV (light-oxygen-voltage) proteins function as light sensors in plants, fungi, and bacteria. RsLOV is unique as the light state is a monomer but the dark state is a dimer. These dimers exchange their monomer units on a time-scale of seconds.
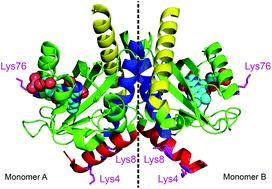
Photochem. Photobiol. Sci., 2020,19, 159-170
https://doi.org/10.1039/C9PP00424F
Raf-like kinases CBC1 and CBC2 negatively regulate stomatal opening by negatively regulating plasma membrane H+-ATPase phosphorylation in Arabidopsis
Raf-like kinases CBC1 and CBC2 negatively regulate phosphorylation of plasma membrane H+-ATPase in guard cells and blue light-dependent stomatal opening.

Photochem. Photobiol. Sci., 2020,19, 88-98
https://doi.org/10.1039/C9PP00329K
A thermostable flavin-based fluorescent protein from Chloroflexus aggregans: a framework for ultra-high resolution structural studies
A new thermostable fluorescent protein is shown to be a promising model for ultra-high resolution structural studies of LOV domains and for application as a fluorescent reporter.
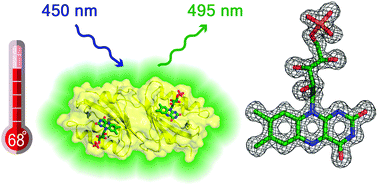
Photochem. Photobiol. Sci., 2019,18, 1793-1805
https://doi.org/10.1039/C9PP00067D
About this collection
This collection forms part of the ‘The World Congress on Light and Life, Barcelona 2019’ issue. Flavin-binding, blue light sensing photoreceptors have become major players in photobiology and optogenetics during the last two decades. They comprise the LOV (Light, Oxygen, Voltage), BLUF (Blue Light sensing Using Flavins) and Cry (Cryptochromes) superfamilies, wide-spread in eukaryotes and prokaryotes. LOV domains are also exploited as alternatives to Green Fluorescent Proteins as well as in a variety of optogenetic applications. Optimized variants and new proteins are continuously explored. Nazarenko et al. introduce a thermostable bacterial LOV protein for advanced structural studies. Magerl and Dick investigate the important issue of LOV domain dimerization investigated by a combined FRET-stopped flow approach with ms time-resolution. Polverini et al. explore the chromophore cavity of a LOV domain by means of molecular dynamics simulations, photoacoustic spectroscopy and point mutations, highlighting the conformational flexibility of key residues and the role of internal water. Many plant and animal pathogenic bacteria have genes for flavin-based photoreceptors. Pezza et al. review the studies on the role of a BLUF photosensor in a dangerous opportunistic human pathogen, underscoring that this protein is a global regulator for responses related to the resistance of Acinetobacter baumannii. Historical observations of blue light responses in plants prompted the exploration of flavin-binding photoreceptors. Hayashi et al. identified molecular partners of the LOV proteins, phototropins, and describe how the interplay with these photoreceptors regulates stomatal opening in response to blue light. Iwata et al. describe how the binding of ATP enhances the light-triggered conformational changes of plant Cry1, so that ATP is an integral part of the functional activation of this photoreceptor. Hammad et al. explore the molecular mechanism by which Cry proteins mediate magnetic sensitivity in plants, highlighting interplay with the photocycle and light-independent reactions.