A thermostable flavin-based fluorescent protein from Chloroflexus aggregans: a framework for ultra-high resolution structural studies†
Abstract
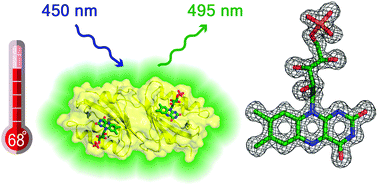
Light-Oxygen-Voltage (LOV) domains are conserved parts of photoreceptors in plants, bacteria and fungi that bind flavins as chromophores and detect blue light. In the past, LOV domain variants have been developed as fluorescent reporter proteins (called flavin-based fluorescent proteins; FbFPs), which due to their ability to fluoresce under anaerobic conditions, fast folding kinetics and a small size of ∼12–16 kDa are a promising reporter system for quantitative real-time analysis of biological processes. Here, we present a small thermostable flavin-based fluorescent protein CagFbFP derived from a soluble LOV domain-containing histidine kinase from the thermophilic bacterium Chloroflexus aggregans. CagFbFP is composed of 107 amino acids with a molecular weight of 11.6 kDa and consists only of the conserved LOV core domain. The protein is thermostable with a melting point of about 68 °C. It crystallizes easily and its crystals diffract to 1.07 Å. Both the crystal structure and small angle scattering data show that the protein is a dimer. Unexpectedly, glutamine 148, which in LOV photoreceptor proteins is the key residue responsible for signal transduction, occupies two conformations. Molecular dynamics simulations show that the two conformations interconvert rapidly. The crystal structure of the wild-type Chloroflexus aggregans LOV domain determined at 1.22 Å resolution confirmed the presence of two alternative conformations of the glutamine 148 side chain. Overall, this protein, due to its stability and ease of crystallization, appears to be a promising model for ultra-high resolution structural studies of LOV domains and for application as a fluorescent reporter.
- This article is part of the themed collection: Photofunctional flavoproteins and UVB photosensors


 Please wait while we load your content...
Please wait while we load your content...