Themed collection Inorganic chemistry approaches to saving critical elements: Recovery, Reuse and Recycling

Sustainability in Ru- and Pd-based catalytic systems using N-heterocyclic carbenes as ligands
This review is a critical presentation of catalysts based on palladium and ruthenium bearing N-heterocyclic carbene ligands that have enabled a more sustainable approach to catalysis and to catalyst uses.
Chem. Soc. Rev., 2021,50, 3094-3142
https://doi.org/10.1039/C8CS00836A
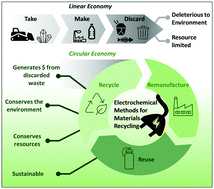
Electrochemical methods for materials recycling
The present review describes electrochemical methods for the recovery of chemical feedstocks from waste materials.
Mater. Adv., 2021,2, 1113-1138
https://doi.org/10.1039/D0MA00689K
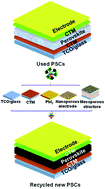
Progress in recycling organic–inorganic perovskite solar cells for eco-friendly fabrication
The review paper has systematically summarized and proposed research-based guidance for recycling organic–inorganic perovskite solar cells and ecofriendly fabrication.
J. Mater. Chem. A, 2021,9, 2612-2627
https://doi.org/10.1039/D0TA07495K
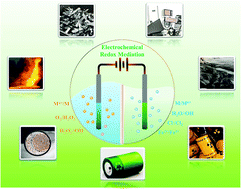
Green electrochemical redox mediation for valuable metal extraction and recycling from industrial waste
This review highlights innovative green electrochemical processes for extracting and recycling valuable metals from industrial waste.
Green Chem., 2020,22, 6288-6309
https://doi.org/10.1039/D0GC02028A
The importance of design in lithium ion battery recycling – a critical review
Product design is an important factor which can control the efficiency and economics of a recycling flowsheet.
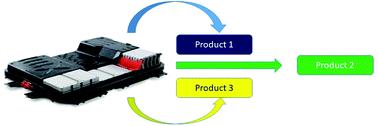
Green Chem., 2020,22, 7585-7603
https://doi.org/10.1039/D0GC02745F
A facile method for the simultaneous recovery of rare-earth elements and transition metals from Nd–Fe–B magnets
A closed loop of recovering rare-earth elements and transition metals from Nd–Fe–B magnets with the total re-use of the electrolyte using a facile electrolysis-selective precipitation procedure.
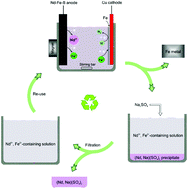
Green Chem., 2020,22, 1105-1112
https://doi.org/10.1039/C9GC03325D
Size-dependent selective crystallization using an inorganic mixed-oxoanion system for lanthanide separation
A unique selective crystallization approach for simple and efficient lanthanide separation has been developed by employing an iodate–sulfate mixed-anion system.
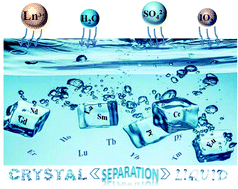
Dalton Trans., 2019,48, 12808-12811
https://doi.org/10.1039/C9DT02387A
A green separation process of Ag via a Ti4(embonate)6 cage
A green supramolecular recovery process of Ag has been developed by using a highly charged anionic Ti4L6 (L = embonate) cage as the extractant.
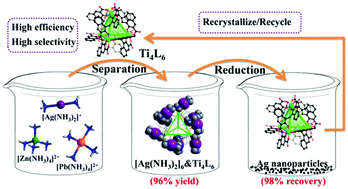
Dalton Trans., 2020,49, 17194-17199
https://doi.org/10.1039/D0DT03214J
Electrochemical oxidation as alternative for dissolution of metal oxides in deep eutectic solvents
Anodic dissolution increases metal ion content in DES, with oxide being oxidised to form semi-stable superoxide species.
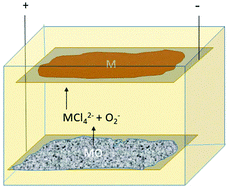
Green Chem., 2020,22, 8360-8368
https://doi.org/10.1039/D0GC03491F
K-gradient doping to stabilize the spinel structure of Li1.6Mn1.6O4 for Li+ recovery
Li+ adsorbent doped with K was prepared and the K entered into the Li1.6Mn1.6O4 (LMO) lattice was confirmed by STEM. DFT calculations further confirmed the K substitution for Li at the 16d sites, which enhanced the stability of LMO.
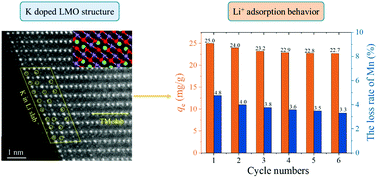
Dalton Trans., 2020,49, 10939-10948
https://doi.org/10.1039/D0DT02405H
Extraction of gallium from simulated Bayer process liquor by Kelex 100 dissolved in ionic liquids
Ionic liquids are shown to beneficially influence the extraction of gallium by Kelex® 100 from alkaline Bayer process liquors.
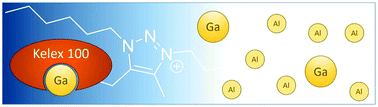
Dalton Trans., 2020,49, 3532-3544
https://doi.org/10.1039/C9DT04623B
Behavior, mechanism and equilibrium studies of Au(III) extraction with an ionic liquid [C4-6-C4BIm]Br2
The first gemini-type benzimidazole ionic liquid ([C4-6-C4BIm]Br2) with two sites of action was synthesized and applied for gold extraction.
![Graphical abstract: Behavior, mechanism and equilibrium studies of Au(iii) extraction with an ionic liquid [C4-6-C4BIm]Br2](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C9DT03860D&imageInfo.ImageIdentifier.Year=2020)
Dalton Trans., 2020,49, 504-510
https://doi.org/10.1039/C9DT03860D
Stability of europium(II) in aqueous nitrate solutions
Investigation of the reduction of Eu3+ and the stability of Eu2+ in aqueous solutions containing high nitrate salt concentrations.

Dalton Trans., 2019,48, 14758-14768
https://doi.org/10.1039/C9DT03139A
The lanthanide complexes of 2,2′-bipyridyl-6,6′-dicarboxylic dimethylanilides: the influence of a secondary coordination sphere on the stability, structure, luminescence and f-element extraction
The secondary coordination sphere contributes to the stability of complexes, the extraction behaviour of the reagents and europium phosphorescence lifetimes.

Dalton Trans., 2018,47, 16755-16765
https://doi.org/10.1039/C8DT03734E
Coordination chemistry of lanthanides in a AOT–CMPO solvent extraction system: UV-Vis and XAFS studies
Synergistic extraction systems using AOT and CMPO were investigated at the molecular level for improved understanding of the lanthanide coordination environment.
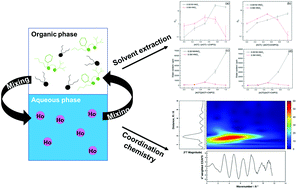
Dalton Trans., 2018,47, 15424-15438
https://doi.org/10.1039/C8DT02957A
Lanthanide extraction selectivity of a tripodal carbamoylmethylphosphine oxide ligand system
Lanthanide extraction selectivity is attained by variation of the ligand structure and extraction conditions for a series of CMPO-based ligands.
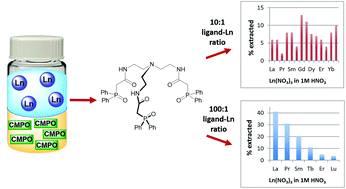
Dalton Trans., 2018,47, 14318-14326
https://doi.org/10.1039/C8DT02239A
Preparation of a mesocellular siliceous foam supported lanthanide-sensitive polymer for the selective adsorption of lanthanides
A novel mesocellular siliceous foam based nanocomposite synthesized for lanthanide selective adsorption is reported.
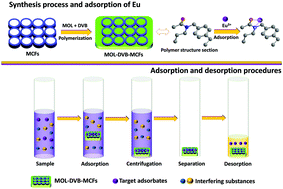
Dalton Trans., 2018,47, 4840-4846
https://doi.org/10.1039/C7DT04255H
Coordination of rare earth element cations on the surface of silica-derived nanoadsorbents
The coordination of REE cations adsorbed on the surface of non-functionalized and complexone-functionalized silica particles was revealed by EXAFS spectroscopy and magnetic studies using X-ray single crystal models as a reference.
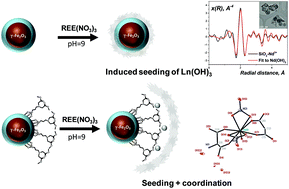
Dalton Trans., 2018,47, 1312-1320
https://doi.org/10.1039/C7DT04388K
An ionic liquid-based extraction system using diglycolamide functionalized macrocyclic platforms for the extraction and recovery of lanthanides
Macrocyclic-DGA platforms are engaged in an ionic liquid-based extraction system. The system exhibits selectivity for middle and heavy lanthanides.
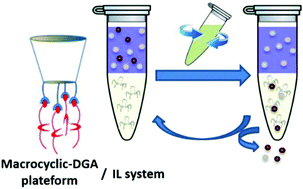
Dalton Trans., 2017,46, 16505-16515
https://doi.org/10.1039/C7DT02797D
Speciation of indium(III) chloro complexes in the solvent extraction process from chloride aqueous solutions to ionic liquids
Indium(III) exists as octahedral mixed complexes, [In(H2O)6−nCln]3−n (0 ≤ n ≤ 6), in acidic chloride media. After extraction, only the tetrahedral [InCl4]− complex is present in the ionic liquid phase.

Dalton Trans., 2017,46, 4412-4421
https://doi.org/10.1039/C7DT00618G
About this collection
Elements from across the periodic table are critical to our daily lives. However, many of the elements used in catalysis, consumer technologies, healthcare technologies and renewable energy solutions are in limited supply, and the processes needed to mine and refine them can come at considerable cost to the environment. Increasing demand in future for these technologies is likely to place considerable pressure on the supply of these elements, requiring new approaches to recover, replace, reuse and recycle them.
This Spotlight collection brings together research published principally in Dalton Transactions and from RSC journals including Green Chemistry, Journal of Materials Chemistry A, Chemical Society Reviews and Materials Advances to highlight emerging science and explore new concepts and opportunities in the recovery, reuse and recycling of critical elements, to ensure the sustainability of our elements for future generations
A companion collection “Inorganic chemistry approaches to saving critical elements: Replacement” brings together research published in Dalton Transactions on sustainable, earth abundant alternatives that will reduce pressure on the supply of critical raw materials.
The collections are guest edited by Eva Hevia (Universität Bern) and James Wilton-Ely (Imperial College London) and are launched in partnership with the Royal Society of Chemistry’s Dalton Division.
This collection will continue to grow and new articles will be added to these themed collections as soon as possible after they are published.
If you would like to contribute an article to this collection, please contact the Editorial Office at dalton-rsc@rsc.org with your proposed topic.