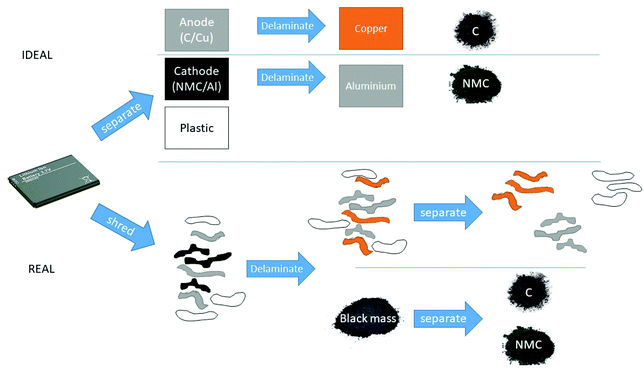
Open Access Article
Open Access ArticleThe importance of design in lithium ion battery recycling – a critical review†
Dana L.
Thompson
ab,
Jennifer M.
Hartley
 ab,
Simon M.
Lambert
bc,
Muez
Shiref
ab,
Simon M.
Lambert
bc,
Muez
Shiref
 bc,
Gavin D. J.
Harper
bc,
Gavin D. J.
Harper
 db,
Emma
Kendrick
db,
Emma
Kendrick
 db,
Paul
Anderson
db,
Paul
Anderson
 be,
Karl S.
Ryder
be,
Karl S.
Ryder
 ab,
Linda
Gaines
ab,
Linda
Gaines
 f and
Andrew P.
Abbott
f and
Andrew P.
Abbott
 *ab
*ab
aSchool of Chemistry, University of Leicester, Leicester, LE1 7RH, UK. E-mail: apa1@le.ac.uk
bThe Faraday Institution, Quad One, Harwell Science and Innovation Campus, Didcot, UK
cSchool of Engineering, Newcastle University, Merz Court, Newcastle upon Tyne, NE1 7RU, UK
dSchool of Metallurgy and Materials, University of Birmingham, Birmingham, B15 2TT, UK
eSchool of Chemistry, University of Birmingham, Birmingham, B15 2TT, UK
fReCell Center, Argonne National Laboratory, Lemont, IL, USA
First published on 20th October 2020
Abstract
Recycling is always seen as an end-of-pipe process returning as much material as possible into a circular economy. There is a growing school of thought that suggests product design should be an important step in the recycling process. While this review is aimed specifically at one technological product, it contains facets that are applicable to the recycling of any complex product. Decarbonisation of energy production necessitates a proliferation of efficient electrical storage and a significant proportion of this, particularly in automotive propulsion, will use lithium ion batteries. The scale of the projected electric vehicle market means that a circular economy model needs to be established while the scale of end-of-life product is still manageable to prevent a build-up of hazardous waste. This critical review investigates the issues of lithium ion battery recycling and discusses the aspects of pack, module and cell design that can simplify battery dismantling and recycling. It highlights not only Green aspects of elemental recovery, but also technoeconomic features which may govern the appropriate direction for recycling. It also shows that as cell design changes, the approach to recycling can become more efficient.
Introduction
To create circular economy for a material it is important to have few components, a lower cost for the secondary process than the primary process, a simple purification flowsheet, valuable components and a collection and segregation mechanism. It also helps when the material has a significant environmental impact if not recycled as this tends to mandate its recycling.These criteria are met by a variety of materials including glass, paper/card, steel, aluminium, plastic bottles, car catalysts and lead acid batteries. These are all well established and mature markets which grow at a manageable rate. The Green Chemistry credentials of these processes for decreasing waste in the environment must not be overlooked; recycling aluminium and steel saves 90% and 60% of the energy of the primary processes, respectively. Substitution and re-use are also a central part of the circular economy hierarchy. However, when new disruptive products and innovations come to the market with large growth potential, a product can rapidly become an environmental issue if a circular economy has not been designed with the product. This was the case in the 90s/00s with the growth in PET bottles, particularly for water,1 and has also been the case with the rapid growth in waste electrical and electronic equipment (WEEE) or the rapid demise of cathode ray tubes.
Disruptive growth in technologies can occur when a new product becomes easy to mass produce, bringing it within the reach of a larger potential market e.g. the internal combustion engine, or when a step change in the performance trajectory of a disruptive innovation reaches a critical inflection point, leading to an incumbent technology being superceded.2 Additionally, environmental regulation can be a driver for requiring technological change in some markets, e.g. the removal of the internal combustion engine, in areas with clean-air mandates.3 The decarbonisation of power production and transport has clear environmental benefits; however, a holistic approach needs to be developed for the materials which enable renewable energy to be harvested, e.g. with photovoltaic devices, electric motors, generators and storage batteries. Step changes in technology cause issues for supply and demand in critical elements, so the change from internal combustion engine to electric vehicles will cause an increase in the demand for metals such as Co, Ni, Li and Nd, and decrease the need for Pt and Pd. Such a step change in technology may enable a circular economy if there is a shortage in the raw material and the economics and logistics enable recycling.4
This critical review highlights just one of these technologies, lithium ion batteries, and tracks the issues that need to be addressed, as well as the lessons that can be learnt from some of the successful recycling industries. It highlights the importance of product design in circular economy and the aspects that can be included to simplify separation.
To evaluate the future of lithium ion battery recycling it is helpful to compare it with the successful lead acid battery market. This ubiquitous product is in most forms of automotive transport as a starter device providing the initiation of combustion. The early history of automotive propulsion was dominated by electric vehicles powered mostly using lead acid accumulators which were invented in 1859. The issues were their poor power density (250 W kg−1) and energy density (40 W h kg−1),5,6 resulting in short ranges. The advent of the internal combustion engine overcame these difficulties and consigned lead acid cells to niche, slow-moving, short-range vehicles. The proliferation of the internal combustion engine is the major contributor to greenhouse gas emissions (estimated at 18%).7 More recent developments of battery technology include the lithium ion battery, which outweighs the lead acid battery in both power density (800 W kg−1) and energy density (180 W h kg−1), making it the cell of choice for modern technology such as electric vehicles (Fig. 1).6,8
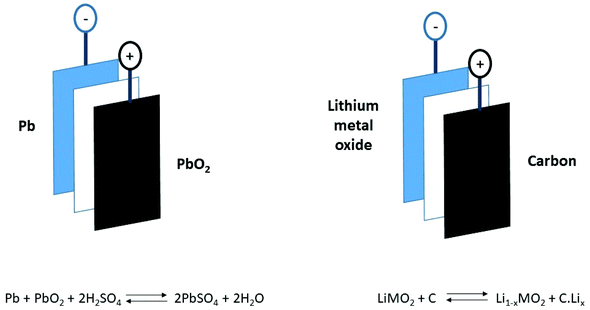 | ||
| Fig. 1 Basic schematic of a lead acid and lithium ion battery.6 | ||
While it has a relatively poor energy density, the lead acid cell has, through numerous iterations, become standardised and is designed for recycling. Modern lead acid batteries are able to reuse >98% by mass of the material.6 This is due in part to the simplicity of their design, where the anode and cathode are Pb and PbO2, respectively. The lead acid battery is self-contained in one unit, not assembled into modules and packs, so it needs no disassembly prior to recycling. Each automotive battery weights 12–21 kg of which lead is more than half the weight which make it economically worthwhile to recycle. The recycling process of a lead acid battery is a simple one: the case is crushed, allowing the sulphuric acid electrolyte to escape, and the lead electrodes are separated from the polypropylene casing and separator by density. The lead is smelted and the polypropylene can be reused in new casings.6
Both Gaines and May et al. state that recycling rates have reached almost 100% in the USA, Japan and most of Europe. This success is because of the incentive to recycle lead acid batteries – it is economical to do so due to the relatively high cost of lead, and the process is an efficient one due to the uniformity of the materials used and battery design.6,9 The materials recycled from the lead acid batteries are then used to manufacture new batteries, thus allowing for closed loop recycling. The success of the lead acid battery circular economy can be easily judged. In the UK in 2018, lead acid batteries accounted for 55% of all batteries collected despite being only 4% by mass of batteries produced. Data on the remaining 96% of batteries are hotly contested. There are a range of different recycling rates cited in the literature,10 however, the recycle rate is significantly lower than that of lead acid batteries due to the complexity of different designs, the low value of materials such as zinc and manganese oxide and the lack of legislation controlling their disposal. Research by Circular Energy Solutions10 critiques much of the existing literature and makes a case that recycling rates are not as pessimistic as the literature would indicate, however there remain challenges. It is important to draw a distinction between lithium ion batteries used in consumer electronics, which are small and easily misplaced, and vehicle batteries, which are large and enter dedicated waste facilities. Recycling processes for lithium ion batteries exist, but the problem lies with their collection rate. Whilst there is a market for viable lithium ion batteries that can be used in second-life applications, it is harder to incentivise the collection of lithium ion batteries for recycling with little economic value, or potentially an associated gate fee. Contrasted with lead acid batteries, this shows the success of a product which has a simple design, a relatively high cost, a low-cost recycling process, a structured collection program and a significant environmental impact if not recycled.
Achieving the same for lithium ion batteries is difficult, due to the more complex cell design and cell chemistry. The lack of any standardisation of cells and the predominance of cells from small portable devices means that initial recycling approaches will be more similar to solid municipal waste, producing streams of lower purity. Homogenisation of cell design and chemistry and the larger fraction of similar automotive cells will enable easier recycling with streams of higher purity and higher value. From a Green Chemistry perspective, it is the scale of the market growth that necessitates the manufacturing and recycling process to be as efficient as possible. As of 2017, there were 3 million electric vehicles in the global stock, which is expected to grow to 125 million by 2030, and 530 million by 2040.11,12 The task facing recycling of lithium ion batteries can be easily understood by comparing it to current recycling markets. Global markets are complex to analyse, so the UK is used as an example, assuming that scrapping rates remain roughly constant at ca. 1 million p.a. reaching end of life. Assuming that by 2040 electric vehicles achieve a market penetration of 50%, approximately 200 kt of lithium ion batteries will reach end of life from an automotive perspective. This may not, however, mean that this is the amount of material that will need recycling as there is significant potential for many of these batteries to reach second life for energy storage, particularly for renewable energy such as wind and solar.13 To put this into perspective, 200 kt is approximately 20 times the size of the current lead acid battery market in the UK. It is therefore clear that new infrastructure will need to be developed to cope with this volume of material and standardise its transport, handling and processing. Depending on process economics, this may then require legislation defining extended producer responsibility for batteries.14 While life cycle analysis has been carried out for the production of lithium ion batteries15 comparatively little analysis of recycling costs and throughputs has been performed. One of the aims of this critical review is to show how product design is an important factor that is often overlooked in Green Chemistry. The recycling metrics can be significantly altered by considering disassembly during the design process. The disassembly of lithium ion battery modules, albeit manually at present, has been shown to produce a high yield (ca. 80%) of total mass recovered in a purer state that was possible using shredded material. The active materials could be short-loop recycled into new electrodes with only minimal performance loss for the anode.16
The growth in lithium ion battery recycling can be judged from Fig. 2 which shows the number of articles and patents on the topic in the past few years. It also shows the actual and projected growth in automotive electric vehicles. This shows clearly how concerted international attention is focussed on the creation of a circular economy for battery materials.
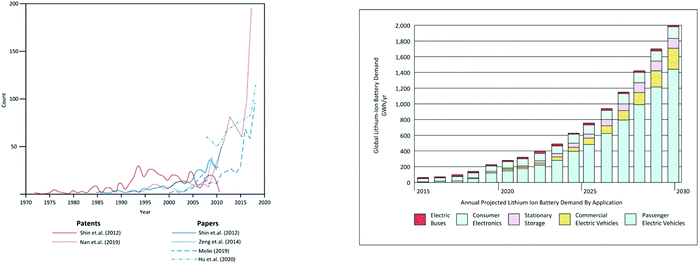 | ||
| Fig. 2 (a) Number of articles and patents on the topic of lithium ion battery recycling (b) Recent and projected growth in automotive lithium ion battery markets. | ||
Lithium ion batteries
Unlike the lead acid battery, the structure of lithium ion batteries is much more complex, with a series of small cells being collected together to make a module and a number of modules are assembled to make the overall battery pack. An automotive battery pack is composed of hundreds or thousands of cells, which not only have to be individually opened but also disassembled from the ensemble. The complex structure and risks associated with electric shock and potential fires make safe dismantling slow and labour intensive. For this reason, many current approaches start with comminution (crushing) in the same approach to lead acid batteries, but this is poor from a Green metric perspective as it requires more steps, more energy and more ancillary processing chemicals.The components of a lithium ion battery pack are shown in Table 1. Each component in a lithium ion battery may consist of different chemistries to those made by, for example, another company within the battery industry. There is a great variation in lithium ion battery cathode chemistries (such as variations of NCA, NMC, LMO, LCO and LFP). In current use, graphite dominates the supply of lithium ion battery anode materials, with low levels of SiOx and silicon being introduced in the high energy cells;17 however, lithium titanate (Li4Ti5O12)18 and more recently TiNb2O7 can be used in lower energy density but high power cells.19,20 One issue for recycling companies is that due to the rapid advances in technology, two versions of the same car model may even have different battery chemistries. Perhaps a desire for quick treatment and simple recycling, given present modest volumes, has led to processing techniques that are chemistry agnostic, resulting in lower purity products. While the hazard labelling of lithium ion batteries is strongly regulated21 the lack of compositional labelling for easy identification, coupled with the simplicity of shredding, is a barrier to more nuanced recycling schemes. Most battery packs contain no information about the chemistry of the anode, cathode or electrolyte, meaning that cells from the different packs need to be dealt with by the same process; this is why pyrometallurgy and comminution are the only acceptable methods of recycling at present. Improved battery labelling would enable different battery chemistries to be separated before processing and would prevent contamination between e.g. NMC and LFP chemistries. The issues of labelling are beginning to be addressed, with the Society of Automobile Engineers (SAE International)22 recently recommending a labelling scheme, and the Chinese Government is also considering mandating labelling of lithium ion batteries.
| Cell component | Materials | Composition/wt% | Cost/% |
|---|---|---|---|
| Cathode active material | Layered structures, e.g. LiCoO2 (LCO)/Li(NixMnyCo1−x−y)O2 (NMC)/Li(Ni1−x−yCoxAly)O2 (NCA) | 22–25 | 65–70 |
| Spinel structures, e.g. LiMn2O4 (LMO) | |||
| Olivine structures, e.g. LiFePO4 (LFP) | |||
| Cathode foil | Al | 4–5 | 1 |
| Anode active material | Carbonaceous materials (graphite, hard carbon), lithium titanate, or silicon-based materials | 24–26 | 8–9 |
| Anode foil | Cu | 3 | 2 |
| Binder | Polyvinylidine fluoride (PVDF)/polytetrafluoroethylene (PTFE)/polyvinyl alcohol (PVA)/carboxymethyl cellulose (CMC)/styrene butadiene rubber (SBR) | 2–3 | 8–9 |
| Electrolyte | Mixtures of ethylene carbonate (EC)/propylene carbonate (PC)/dimethyl carbonate (DMC)/ethyl methyl carbonate (EMC)/diethyl carbonate (DEC) + additives e.g. fluoroethylene carbonate (FEC)/vinylene carbonate (VC) | 10–12 | 1 |
| Conductive additive | Acetylene black (AB) | 1 | 0.1 |
| Conductive salt | LiPF6 | 1.5–2 | 8 |
| Separator | Polyethylene (PE)/polypropylene (PP) | 4–5 | 4 |
| Cell case | Varies (metal or laminate) | 4–6 | 4 |
Table 1 shows a number of different chemistries for each component, including a rough idea of the cost of each component. This is clearly only a guide, as cost depends on the chemistry of the cathode material, the type of binder, the solvent and additives. Crucially, the main drivers for cost are scale and purity, which can only be roughly estimated here, but other similar studies have similar breakdowns.23
The following equations denote the half redox charge and discharge reactions in terms of Li ion movement in the anode and cathode, respectively:
| LixCn(s) ⇌ xLi+(soln) + xe− + nC(s) | (1) |
| Li1−xMO2(s) + xLi+(soln) + xe− ⇌ LiMO2(s) | (2) |
The importance of product design in recycling efficiency
This is an often-overlooked concept in circular economy discussions. Recycling is seen as an end of pipe activity, processing a device which has been developed for optimal performance often without thought of dismantling or recycling protocols. Lead acid cells represent a technology which is easy to open (mechanical crushing), rapidly liberating a significant proportion (ca. 60% by mass) of metal per unit with a suitable value which can be separated from the other components due to significant density differences. By contrast, alkaline zinc–carbon batteries account for about 80% of cells produced worldwide (over 10 billion units p.a.) but each contains 25% steel casing, and only 15% of Zn, and 17% of MnO2. The similar densities, 7.14 and 5.03 g cm−3, coupled with the low cost of virgin material, makes recycling less economically viable than lead acid batteries.Macroscopic design issues
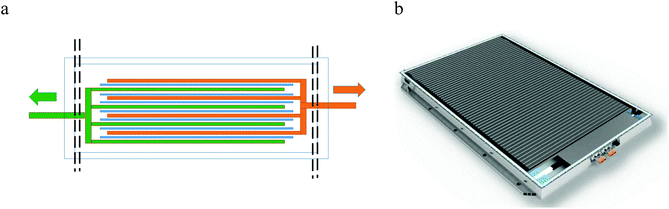
For automotive applications, battery packs need to be both power and energy dense, which can only be achieved by aggregating cells into modules, and modules into packs.31 Increasing the number of cells in a module decreases the ratio of active material: cell case and complicates the issue of opening cells. A Tesla S85 Mk 1 battery pack, for example, contains 7104 cylindrical cells, whereas a Nissan Leaf Mk 1 22 kW h battery pack is made up of 192 pouch cells, and a BMW i3 Mark 1 22 kW h battery pack contains 96 prismatic cells. This is shown pictorially in Fig. 3. The active energy storage mass of the pack can be calculated as 64%, 60% and 82% of the total pack mass for the Tesla, Nissan and BMW respectively.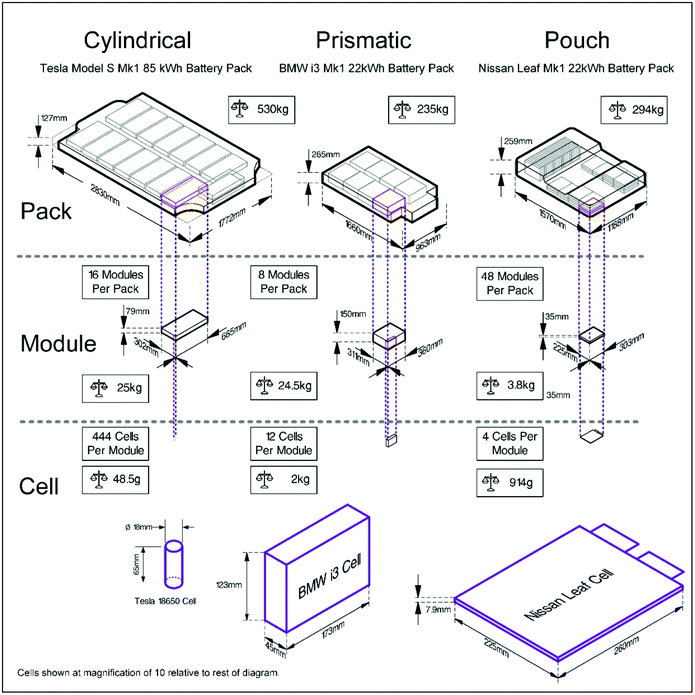 | ||
| Fig. 3 Different types of battery cells and how they are organised to form modules and packs.32 | ||
One of the main issues is the way in which the cells, modules and packs are assembled. The cells themselves are hermetically sealed and the modules and packs are often glued together with adhesives.32 This provides rigidity, but means that they can often only be dissolved in molecular organic solvents. This precludes disassembly as a viable recycling method due to the time and solvent requirements. The assembly of packs and modules is probably the largest barrier to disassembly and hence efficient cell dismantling and recycling. Marshall et al. describe the complex steps required to separate cells.16 While the disassembly approach is more successful at recovering more material, and in a purer state, this is naturally at the sacrifice of the speed at which material can be processed – which is limited by the pack, module and cell opening. The structures are clearly established for safety and potentially cell longevity, but at the expense of recycling efficiency. When dismantling is slow and costly, the only method of recycling becomes pyrometallurgy, which is expensive and inefficient. Recycling is therefore in a “Catch 22” situation, where cell and pack design controls recycling strategy. The lack of binding legislation or policy does not enforce improvements in recycling efficiency and this in turn does not influence cell design.
The ideal recycling process
Theoretically, the aim of a recycling process is to divide the constituents of a device into chemically pure, distinct phases. Separation of components in a mixture depends upon a difference in the properties of the components which can be utilised to bring about a separation. These differences include size, density, wettability, magnetism, redox behaviour, surface charge, solubility, appearance, phase change, adsorption, and combustion. The ability to bring about a separation depends upon the relative affinity of the components to the property being distinguished. The separation factor, Ki, of a specific phase in a separation step could be expressed mathematically as: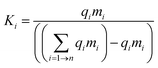 | (3) |
| Component | Solvent | Issues | Advantages |
|---|---|---|---|
| Binder | Organic solvent | Higher cost, flammability | Targets binder and does not etch metal |
| Collectors | Oxidising agents | Aq. acids – poor LCA | Lower cost |
| Aq. base – low cost | |||
| Metal oxide | Acidic solutions | Reactivation of cathode material | Lower cost |
| Carbon | None | Insoluble | Easily reactivated |
A more ideal scenario would be to dismantle the pack and remove the liquid phase (2 components), followed by splitting the solids into individual bins of electrodes, packaging and separators as shown schematically in Fig. 4. Each electrode bin would only contain the current collector, active material and a binder. Use of binder which was soluble in water or a Green solvent such as alcohol would enable simple separation of the current collector from the active material without significant use of ancillary chemicals.33 Separating the powdered active material from the current collector sheet can be done on size. This ensures the minimum number of components in each bin and the largest possible selectivity coefficient. Li et al. used two water miscible binders; a carbon black (CB)/carboxymethyl cellulose (CMC)/styrene butadiene rubber for the anode and a CB/CMC/PVDF binder for the cathode. The normal PVDF binder would be cast using N-methyl-2-pyrrolidone which is toxic and expensive. Water with binders which can be dispersed in aqueous solutions were used to produce a cell which showed similar charge–discharge properties. The water-soluble component of the binder (CMC) enabled the cell to be recycled using water. The recovered NCM523 cathode material was re-lithiated, and shown to have a performance similar to the unused material.34
Macroscopic recycling issues
The first design issue that needs to be addressed is how to open the pack, module and cell easily. Clearly the outer pack design needs to be as robust as possible, so it does not fail in service, but this does not preclude mechanisms which are easier to open. Metallic tools need to be avoided to decrease the possibility of shorting the cell and igniting the contents.35 This could be a particular issue for prismatic and cylindrical cells, which are already used in primary battery products.The importance of a simple disassembly mechanism has been highlighted by several authors and some attempts have been made to automate the opening of pouch cells.36–38 Many groups agree that module and pack disassembly tasks should be carried out using smart robots.39 While this can be achieved at pack level, it is complicated by the myriad pack designs and fixings and glues that are used to assemble packs.40 While pack shapes and configurations will clearly change with each manufacturer, standardisation in fixing type would simplify disassembly as it would only require one tool. It should be noted that for robotic disassembly, flexible cables present a challenge, and a move to solid busbars that are fixed in a predictable position (vs. a flexible cable) may simplify disassembly.
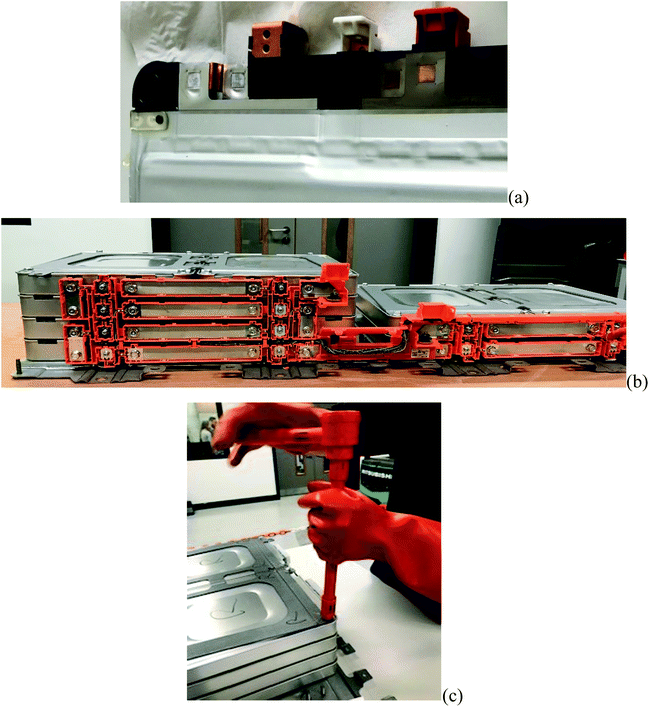
Pack and module designs vary significantly, even within a manufacturer's own fleets. It is common, regardless of the form factor of the cell, to assemble groups of cells into modules but the variety of the number of cells and the series/parallel configurations is broad (as indicated in Fig. 4). Typically, regardless of the arrangement, cells in a module are permanently affixed to one another and are not intended to be broken down as part of a servicing activity. A total of nine joining technologies are identified,41 all of which are, in the sense of serviceability or disassembly, permanent processes, and attempts to disassemble these joints are likely to be a destructive process. An example of pouch cell tabs welded to copper busbars in a 2S2P module for a first-generation Nissan Leaf module is shown in Fig. 5(a).
Connections between modules are typically more serviceable and may employ technologies such as threaded or torqued connections or bespoke, mechanically constrained push-fit connections. The repeatable functionality of these connections makes the modules both more easily replaceable in a service situation and simpler to disassemble at the end of life. Fig. 5(b) shows solid busbar interconnections between modules of a first-generation Nissan Leaf held in place by threaded bolts. The mechanical fixings between modules are often equally serviceable, Fig. 5(c) shows manual disassembly of a sub-pack architecture which would more easily lend itself to automation.
At cell level, standardisation is more difficult to achieve since cylindrical, pouch and prismatic cells are commonly used. Recent studies have shown that the polymer separator between the electrodes can be used as a method to separate the active components of the cell. An apparatus built by Li et al. used a vacuum conveyor equipped with pinch grips and a series of skimmers to separate the anode from the cathode and the separator.42 A Z-fold uses a single sheet of separator wound alternately between the anode and cathode in a cell. As the Z-fold is flattened out the anode and cathode will automatically be partitioned onto opposite sides of the separator. This is shown schematically in Fig. 6. It could work equally well with prismatic, cylindrical or pouch cells, but automated methods of cell opening need to be built into the design. It should, however, be noted that these separators can be very fragile, particularly at end-of-life (EoL), and often rip during disassembly. Separators are also becoming more complex; early versions were homopolymers of polypropylene, but later ones are composites or mixed polymer (PE/PP) and some even contain ceramic layers, complicating the recycling process.
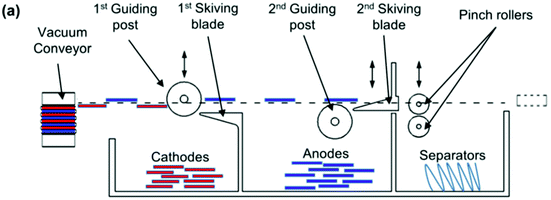 | ||
| Fig. 6 Schematic diagram of a prototype device for separating anodes, cathodes and separators from a lithium ion battery.42 | ||
An alternative approach to dismantling could also be achieved by simply changing the geometry of the electrode connector tabs which connect the electrodes of the same polarity together. This is beginning to be done by some manufacturers. The incorporation of a failure mode, probably cut as notches post EoL, as shown in Fig. 7(a) (dashed line) could enable simple separation of anode and cathode stacks and enable the polymer components to be easily segregated.
A recent development by Chinese battery manufacturer BYD has developed long, thin cells called blades, which fit into a grid and can impart structural strength to the battery pack. Although details are currently unpublished, this could remove the need for separate modules and glues, enabling simple disassembly and exchange for cells when faults develop. The manufacturers claim that the optimised pack structure enables an increase of 50% in space utilisation and leads to safer operation of cells.43 This shows that battery development is a fast-moving subject.
An important aspect of pack design is the standardisation of fixings. Each manufacturer places pack fixings in different locations which need different tools to open them. Standardisation of pack opening is fundamental to cell dismantling.40
Comminution vs. dismantling
Currently, the over-riding factor with lithium ion battery use and recycling is safety. The potential consequences of the battery chemistry coming into contact with moisture necessitates hermetic sealing of the cell, which naturally complicates end of life processing. To date, the majority of processes have either shredded the cell under an inert atmosphere or used high temperature pyrometallurgy.Shredding the cells effectively dilutes all the constituents, which then need to be physically separated by froth floatation, electrostatic, magnetic or density separation techniques in combination with wet chemical processes. All of these have inherently low selectivity coefficients leading to products with lower purity, lower value and necessitate extensive purification to re-enter the manufacturing circle.44
Pyrometallurgical processing is currently seen as the pragmatic approach to recycling as it already functions for a range of disparate materials; however, it is only economically viable through implementation of significant gate fees to process the material. It is fast and generally safe but will lose the volatile elements and have a higher energy and ancillary input. Nonetheless it will lead to a higher purity of the highest value metals. From an LCA perspective, opening and separating the cell into the electrodes, separators and electrolyte and delaminating the separated electrodes will require fewer steps and potentially fewer ancillary chemicals than shredding. This naturally has the barriers that the cells are not easily opened and there are safety issues with the current cell designs.
From a pragmatic perspective, the future of lithium ion battery processing will probably require a mixture of processing, including pyro- and hydro-metallurgical methods blended with mechanical separation, as the design enables it. This will lead to different grades of material which probably originate from different sources. Small cells originating from mobile devices currently dominate the lithium ion battery market but as the automotive sector expands this will ultimately govern the main approach to cell design and recycling. This will probably result in different process economics, with gate fee processing and smaller scale pyrometallurgical approaches dominating initially, and as economies of scale permit larger plant and simpler cell design, this may negate the need for processing tolls.
Cell evolution
Cell evolution is a dynamic process and numerous improvements to cell chemistry and design have been made to improve safety45 and performance.46 There is an on-going need to optimise energy and power density, which was initially achieved through cell chemistry and later by active material morphology. The evolution of cells in terms of their chemistry has been reviewed.47,48 The cell design, and hence cell manufacturing process has evolved with the requirements for different applications. There is always a trade-off between energy, power and life-time. Factors such as the current collector thickness, active material particle size, electrode composition, density and thickness, separator and tab configuration can all affect the cell resistivity, power performance, volumetric and gravimetric energy density.46 For example in consumer electronics, high energy small pouch or prismatic cells are used. Here the pouch cells are often stacked using a Z-fold configuration with parallel terminal tags – this cell design provides an ease for automated manufacturing of the cells. To maximise the energy density, thinner current collectors, tags and separators are utilised where possible, and high loadings of active material are coated upon the current collectors, with low porosities. For safety reasons separators are often either slightly thicker to further separate the anode and cathode, or coated in a thin ceramic layer to help prevent short circuiting. For larger cell formats Z-fold configurations are more difficult, and therefore stacking or winding is more prevalent, for example in electric vehicle cell manufacturing.49 Stacking with individual separator sheets leads to an excess of separator, which is then incorporated into the pouch sealing, reducing the excess, but providing added complexities in the separation of the anode and cathode from the cell if using disassembly methods. In high power applications, the electrons need to be transported from the point of redox reaction in the electrode to and through the current collector much quicker, and therefore thinner electrode coatings, thicker current collectors and tabs are utilised, this reduces the energy density of the cell. The separator however despite safety issues is as thin and porous as possible, as often the separator exhibits the largest charge transfer resistance within the cell. In addition, cell designs may be modified so that the current density (and hence heat) distribution is more even at the high currents and opposing, larger tabs are often utilised for high power cells in, for example, hybrid electric vehicles.50,51As mentioned briefly above, another vital aspect of lithium ion battery design is safety. Resistive heating during charging and discharging, as well as thermal run away and over-charging, can damage the cell and lead to fires. Hundreds of fires occur each year at waste and recycling plants, with up to one third caused by lithium batteries, mostly through the act of crushing the cell. Numerous design features are added to improve cell safety45,52 but changes in cell design can lead to high profile catastrophic failure such as the Samsung Note 7 issues in 2016, or the Boeing 787 Dreamliner in 2013.53 The formation of lithium dendrites on the anode, decomposition of the cathode or the electrolyte releasing gases, and damage to the separator can all lead to cell failure. There are numerous additives which can be incorporated, including shear thickening electrolytes to limit puncture damage, strengthened separators to stop dendrite penetration, redox shuttle additives to stop overcharging, and flame retardants in the electrolyte and separator. All of these add to the complexity of the cell and will have an impact on the recycling efficiency.45
One of the largest factors affecting energy density is the electrode configuration and packaging type. As shown in Fig. 3 the main cell designs are cylindrical, prismatic and pouch. The rolled 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 cylindrical cell has a higher energy density (248 A h kg−1) than the pouch or prismatic cell (140 A h kg−1). An important aspect of module and pack design is the ability to dissipate heat. Smaller cells can aid heat dissipation but can make disassembly and recycling more complex.
650 cylindrical cell has a higher energy density (248 A h kg−1) than the pouch or prismatic cell (140 A h kg−1). An important aspect of module and pack design is the ability to dissipate heat. Smaller cells can aid heat dissipation but can make disassembly and recycling more complex.
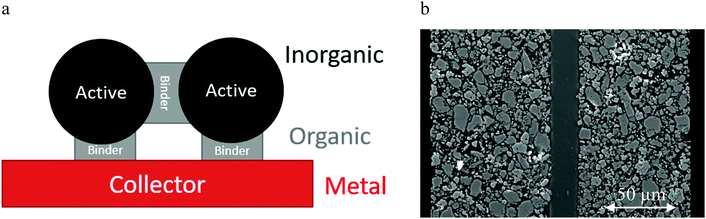
Microscopic recycling issues
When the electrode materials, which contain most of the inherent value, have been separated from the electrolyte, separator and casing, they need to be split into their constituent components. The main barrier for recycling is being able to split the interface between the current collector and the active material, as shown schematically in Fig. 8. The importance of the binder is often overlooked. There are two failure mechanisms: either breaking the adhesive bond between the current collector and the active material, or by breaking the cohesive bond between the active particles.Organic solvents can be used as lixiviants to target the binder, whereas basic lixiviants will etch aluminium. Aqueous oxidising acids can oxidise both copper and aluminium while also dissolving metal oxides. Etching of collector materials causes problems for downstream processing, as contamination of metal oxides with either copper or aluminium can affect the performance of subsequently reformed cathode materials. However, careful choice of the water-soluble additives and optimisation of the separation process can result in well separated and pure waste streams with green solvents.16 Short loop recycling of the reclaimed anode and cathode is possible with these separation methods. In water-based systems, graphite can be re-processed and re-used easily, however often a relithiation step (or healing) is required of the cathode before it has a useable specific capacity in a remanufactured cell.54
An additional simplification is to look to new electrode designs which negate the use of binders between the current collector and active material. This is not a new concept, primary alkaline cylindrical designs have been doing this for some time and are using compacted active powders on current collectors as their electrodes,55 however this has not been yet transferred to lithium-ion systems, and may offer future research avenues.
Binder degradation in aged electrodes can also interfere with mechanical integrity. Studies by Demirocak et al. revealed that mechanical degradation is due to a reduced crystallinity of PVDF, as indicated by a decreasing hardness and modulus of elasticity with age.60,61 This can affect the electrode by reducing the adhesiveness of the binder to the active material and current collector (i.e. loss of contact).62 Therefore, characterising the binder distribution and adhesion is crucial when it comes to designing a recycling scheme, as this can affect methods that target the current collector interface for separation. Numerous other binders have been tested but there is a dichotomy between in-service stability and end of life solubility.57,63 From a life-cycle analysis perspective, design of a strong and stable, yet soluble binder is an important goal. An alternative perspective would be to develop electrodes which do not require the use of a binder.
The recycling process
The recycling process itself will have a number of inputs, including transportation, energy, ancillary chemicals, waste treatment, labour and plant. The outputs will be various bins of material of differing purity and the value of the outputs will need to exceed those of the inputs, or a processing (gate) fee will need to be applied. Gate fees are only really viable where legislation mandates a material which is particularly hazardous to handle, e.g. nuclear material or asbestos. They are currently applied to processing lithium ion batteries, but for a commodity as large-scale as cars, can result in undesirable behaviours such as unlicensed disposal, encouragement of environmental crime, etc. if they are excessive.It is not the aim of this review to extensively cover the recycling methods as this has recently been published.32,64,65 Instead, this review shows that the design necessitates given recycling processes, with their associated efficiencies and costs. In this section the general advantages and disadvantages of pyrometallurgy and hydrometallurgy are discussed together with the aspects of cell design that tend to necessitate particular recycling approaches. It has been recently shown that recycling shredded material can potentially return material back to the manufacturing process with cost savings of approximately 20% compared to never used materials. The prior separation of electrode materials followed by delamination can increase the cost savings by up to 70%, producing higher purity material.66
To begin recycling, the batteries must be discharged to a safe level in order to avoid short circuiting and self-ignition during dismantling.32,67 The majority of batteries are, however, either shredded in an inert atmosphere or over-discharged. Over-discharging can result in unwanted side reactions, such as anode dissolution into the electrolyte and the formation of copper dendrites, resulting in contamination of material streams (such as the cathode).32,68 Furthermore, it is argued that discharging can add costs to recycling processes. Low voltage cells can be discharged using salt solutions, where adequate control of electrolysis can be achieved. However, for electric vehicle battery packs with high voltages, this method is not viable unless the pack has been dismantled. Other methods, such as the use of halide salts, have been utilised for safer discharging, however corrosion at the battery terminal ends can occur.32,69 After discharge, the battery packs are manually dismantled.32,70 Since these packs are heavy and of high voltage, specialised tools, as well as qualified employees, are needed.32,71
The lithium salt used is soluble at a concentration of approximately 1 mol L−1 and has a large anion to allow for good dissociation (e.g. LiPF6). A variety of additives include fluoroethylene carbonate (typically <5 wt%), which are included to improve long-term cycling and reduce self-discharge.28,73 Electrolyte stability plays a key role in the longevity of the battery by the formation of a stable solid electrolyte interface (SEI) layer.74 Numerous studies have focussed on the scarcity of lithium75–77 as the main reason for recovery and highlighted the importance of recovery, however, simple analysis of the cost of the electrolyte shows that the anion is the major cost and phosphorus is as scarce as lithium. Most recycling processes lose PF5 and recover lithium as a carbonate salt, but it is important to recover the fluorine component due to the value and the avoidance of treating fluorine-containing waste, particularly in the gas phase.
Current recycling processes
Worldwide lithium ion battery waste in 2020 is estimated to be approximately 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes but the majority of this (>80%) originates from portable electronics.78 The lack of standardisation on pack and cell level coupled with the complexity of storage, transportation and handling of EoL batteries all contribute to the increased cost and decrease the incentive to recycle.9,78 Battery recycling is being carried out in numerous demonstrator and pilot scale processes.65,79 Some have modest capabilities, while others are semi-production scale (5000–7000 t per year). A report by Wang et al. suggested that even relatively small plants could break even financially, processing as little as 170 t per year using hydromechanical processing, although this depends on having a high cobalt content and a high cobalt price.80
000 tonnes but the majority of this (>80%) originates from portable electronics.78 The lack of standardisation on pack and cell level coupled with the complexity of storage, transportation and handling of EoL batteries all contribute to the increased cost and decrease the incentive to recycle.9,78 Battery recycling is being carried out in numerous demonstrator and pilot scale processes.65,79 Some have modest capabilities, while others are semi-production scale (5000–7000 t per year). A report by Wang et al. suggested that even relatively small plants could break even financially, processing as little as 170 t per year using hydromechanical processing, although this depends on having a high cobalt content and a high cobalt price.80
Some of these facilities are already dealing with Co and Ni recycling from other sources. Some hydrometallurgical plants in China have capacities in excess of 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t per year. China's largest lithium ion battery recycling company, BRUNP, recently announced an investment of $178 million for a new plant in the Hunan province with a capacity of over 100
000 t per year. China's largest lithium ion battery recycling company, BRUNP, recently announced an investment of $178 million for a new plant in the Hunan province with a capacity of over 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t per year which was due to open in February 2020.81 BRUNP is a subsidiary of the Chinese battery manufacturer CATL and, as a consequence of the economics of a circular economy, manufacturers and recyclers are increasingly collaborating and co-locating.
000 t per year which was due to open in February 2020.81 BRUNP is a subsidiary of the Chinese battery manufacturer CATL and, as a consequence of the economics of a circular economy, manufacturers and recyclers are increasingly collaborating and co-locating.
The two main types of recycling currently involve pyrometallurgy or hydrometallurgy. Pyrometallurgy utilises furnaces and a reductant to produce metal alloys of Co, Cu, Fe and Ni.32,70 Its main advantages over hydrometallurgy include the ability to deal with different battery chemistries and cell types, which negates pre-treatment (mechanical or pre-discharging), and its relative safety due to lack of handling.82 The main disadvantages are high operating costs due to high energy input, a reduced recycling efficiency, as Al, Li and Mn are lost in the slag and downcycled into the concrete industry, and the emission of harmful gases.26,32,70,82–84 All plastics are also lost in the process and harmful gases must be scrubbed.26,32,84 Initially, pyrometallurgical processes were favoured due to their existing use with Ni and Co based alloys. This was made possible as capital investment had already been made in the plant. The main commercial pyrometallurgical lithium ion battery recycling processes are operated by Umicore AG & Co. KG in Belgium and Sumitomo-Sony. At best, this process can only recover 50 wt% of material for reuse in batteries. In addition, since cobalt is the main element of recovery, the economic efficiency of the Umicore battery recycling process heavily depends on cobalt content and prices.85Umicore have anticipated a large market growth in electric vehicle (EV) battery recycling by 2025, and have invested £25 million in the Belgium plant.86 At present day, the maximum processing capacity of Umicore is 7000 tonnes p.a., equivalent to approximately 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 28
000 to 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 EV batteries although larger expansions are planned. In comparison, companies such as Akkuser (Finland) and Accurec (Germany) process 1000 and 1500–2000 tonnes of battery material p.a., respectively.70,87 With the predicted increase in EV production (up to 125 million EVs in the global stock by 2030) it is obvious that more facilities are needed to cope with the increasing demand.11,88,89 The ultimate capacity required for recycling is subject to debate but a conservative estimate that 8 million tonnes p.a. will reach EoL by 2030 suggests that capacity cannot be met by pyro-metallurgical processing alone. Given the structure of the recycling industry it is probable that a significant proportion will be with hydrometallurgy.
000 EV batteries although larger expansions are planned. In comparison, companies such as Akkuser (Finland) and Accurec (Germany) process 1000 and 1500–2000 tonnes of battery material p.a., respectively.70,87 With the predicted increase in EV production (up to 125 million EVs in the global stock by 2030) it is obvious that more facilities are needed to cope with the increasing demand.11,88,89 The ultimate capacity required for recycling is subject to debate but a conservative estimate that 8 million tonnes p.a. will reach EoL by 2030 suggests that capacity cannot be met by pyro-metallurgical processing alone. Given the structure of the recycling industry it is probable that a significant proportion will be with hydrometallurgy.
The economics of the process are still governed by recovery of the cathode material but hydrometallurgy does have the advantage that Li, graphite, Mn and Al can all be recovered and the energy costs are comparatively lower than pyrometallurgy.73,82 The challenge facing recyclers is to reactivate the active material so that it performs the same as virgin material. Numerous approaches have been shown to be effective, although few life cycle assessment (LCA) or technoeconomic assessment approaches have quantified the efficacy of these.91
Little data exists in the open literature on hydrometallurgical processing metrics but Table 3 lists some of the hydrometallurgical processes for which loadings are available and approximate relative processing costs can be estimated (see ESI† for method and details). This shows that significant differences in efficiency can arise depending on the specific processing conditions. The relative costs and value for each process are in themselves little value other than to display the factors affecting them (time, temperature and solid to liquid ratio) and the relative change in the process economics. As expected, the low temperature, high S/L and shorter times had a lower relative cost. The addition of a reducing/oxidising agent also has a significant effect on process kinetics and economics.32
| Literature reference | Input material | Acid (M) | Redox agent (M) | T (°C) | t (h) | S/L (g L−1) | Relative cost | Relative value |
|---|---|---|---|---|---|---|---|---|
| Kim et al.92 | Cathode black mass | H2SO4 (2) | H2O2 (2.13) | 60 | 2 | 100 | 10 | 2.9 |
| Dutta et al.93 | Cathode black mass | H2SO4 (2) | H2O2 (4.26) | 30 | 3 | 75 | 22 | 0.36 |
| Gao et al.94 | Cathode black mass | Formic (1) | H2O2 (6) | 60 | 1 | 50 | 55 | 1.6 |
| Huang et al.95 | Cathode black mass | HCl (6.5) | H2O2 (6.4) | 60 | 2 | 200 | 14 | 1.5 |
| Prabaharan et al.96 | Calcined black mass | H2SO4 (2) | Electrolysis, 400 A m−2 | 25 | 3 | 75 | 10 | 1.1 |
| Hu et al.97 | Calcined black mass | H2SO4 (3.5) | — | 85 | 3 | 200 | 2 | 2.5 |
| Yang et al.98 | Calcined black mass | H2SO4 (4) | H2O2 (2) | 90 | 2 | 25 | 43 | 3.0 |
| Barik et al.99 | Calcined black mass | HCl (1.8) | — | 50 | 1.5 | 200 | 1 | 1 |
It is interesting to note the extent of the relative cost of each process per kg of material. The relative value is less easy to assess as there are differences in the input materials studied, but there is also variation in the yield of each component combined with the form in which that element is obtained. In general, the oxides are significantly more valuable that the metals. Producing pure metallic Co, Mn and Ni from the cathode material can recover less than 5% of the cost of the cell, whereas recovery of pure, active metal oxide can recover approximately 50–60% of the cost of the components. Clearly, the most important factors in this calculation are the yield, purity and activity of the material in each cell. Nevertheless, the data in Table 3 show that economics of hydrometallurgical processes can vary quite significantly depending on the operating conditions.
The most common reagent used is H2SO4, usually in combination with H2O2. Sulfuric acid is used as it is a waste product from a variety of processes including flue gas capture and lead acid battery recycling. It is accordingly the lowest cost lixiviant and the effect on relative cost of changing from H2SO4 to formic acid can be seen in Table 3. Other reagents include HCl, HNO3 and H3PO4.32,100 The use of H2SO4 and HCl releases gases such as sulphur trioxide and chlorine, respectively, and these need to be scrubbed from the air.101 Organic acids (such as citric, oxalic, ascorbic and maleic acid) have been studied to circumvent this,82,100 but an issue is that of selectivity to the metals and the ability to work with different battery chemistries.69,82
In addition to the economics of the process, recovery efficiency and the rate at which material can be processed (space time yield, STY (yield/(time × volume)) are important parameters. Again, these data are not widely available in the literature, but data gathered as part of the ReLiB project are summarised in Table 4. These data show significant differences in relative costs with aqueous lixiviants. It can also be seen that step changes in STY can be achieved using solvomechanical processes. A recently patented methodology developed as part of the ReLiB project can delaminate a 20 × 20 cm anode in less than 20 s, enabling fast, efficient, selective separation just using water with some materials.
| STYa/kg m−3 min−1 | Costb/kg−1 | Efficiencyc/% | |
|---|---|---|---|
| a Electrode material delaminated. b Energy and solvent cost relative to HCl. c Percentage of material recovered in non-contaminated bins. | |||
| Cathode | |||
| 1.0 M H2SO4 | 0.045 | 1.29 | 67.2 |
| 1.0 M HCl | 0.20 | 1 | 61.1 |
| 0.5 M NaOH | 0.66 | 0.56 | 99.0 |
| High power solvomechanical | 2.84 | 0.14 | 99.5 |
| NMP | 0.033 | 70.2 | 96.9 |
| Anode | |||
| Low power solvomechanical | 0.071 | 0.2 | 99.0 |
| High power solvomechanical | 2.84 | 0.12 | 99.4 |
Given that a typical car battery contains 65–70 kg of active cathode material, the STY data in Table 4 show that separation could be achieved in 1 m3 in about 30 min if the pack and cell were easy to disassemble.
Associated issues with recycling lithium-ion batteries
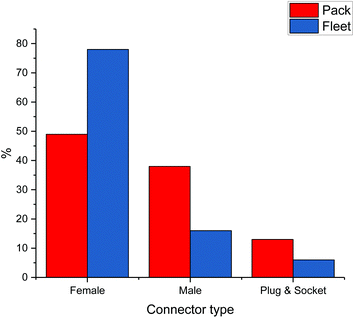
An important issue associated with EoL batteries is reuse. Automotive packs are thought to have an estimated lifetime of between 8–12 years, depending on charging history, but even at the end of this period they could potentially have up to 20 years storage capacity, which will become a growing market with increased production of electricity from renewable sources. Gateway testing is a diagnostic process whereby the health of a battery pack can be determined. This is another example of where standardisation in batteries is important and most manufacturers have different connectors. Connectors vary not only in type but in size and shape and relative permanence. The complexity of the disassembly process is also a function of the number of operations required in order to reduce the battery to the required scale, thus the variability of the intermodular connections is complicated by the number of connections in any particular pack. For the purposes of this work intermodular connections have been simplified into three categories: male and female threaded, and plug & socket (although clearly there will be broad variations within each case). Fig. 9 shows a study of the proportions of packs employing each of these three connection types from the perspective of the types of pack and also as the fleet as a whole. The present lack of standardisation throughout the (H)EV fleet in cell and module interconnections means that the tooling provision requirements for pack disassembly becomes somewhat reliant on the volatile trends in particular model sales.The issue of standardisation is also encountered with labelling. Most battery packs contain no information about the chemistry of the anode, cathode or electrolyte, meaning that all packs need to be dealt with by the same process; this is why pyrometallurgy and comminution are the only acceptable methods of recycling at present. The presence of different elements and binders can affect the efficiencies of separation and lead to contamination. For example, Fe, Cu and Al can all affect the ability of Ni, Co and Mn to be recycled into cathode material.32,78 Despite this, Umicore claim to be able to recycle lithium ion batteries of all types using their pyrometallurgical process.102 Not only are there recycling difficulties with electrode chemistry, but there are also difficulties on a battery pack-level when it comes to disassembly. As shown in Fig. 3, the packs of different models are vastly different which complicates their safer, faster and more efficient disassembly using robotics. Design difference between manufacturers in accessing screws and cables is an ongoing issue and cries out for standards which have been realised in the lead acid battery market.9,78 Designs for recycle should also factor in that packs may be 30 years old before EoL recycling, and the materials used for labels and fixings need to be durable and not corrode/erode.
The prevalence of fluorine in the component materials of used batteries presents additional complications.33 HF is a known and unwelcome product of electrolyte degradation on exposure to humid air.103 Moreover, the common cathode binder PVDF is an excellent low-temperature fluorinating reagent for metal oxides, potentially complicating low temperature cathode recovery and regeneration routes.104 The defluorination of the lithium ion battery would therefore be highly beneficial from a recycling perspective. Chemical additives employed to improve the performance, longevity or safety of lithium ion batteries can also form up to 5% of the electrolyte and are generally regarded as commercial secrets.28,105 This makes detailed knowledge of their potential toxicity impossible, presenting a further challenge to recyclers.
At present, there is little incentive to recycle lithium ion batteries compared to the lead-acid batteries. Tytgat states that the minimum recycling efficiency, as set by the EU Battery Directive, is only 50% for an entire lithium ion battery pack. Since the cells in a battery pack can contribute less than 50% of the mass, it could be argued that they need not be recycled, especially if there is no valuable material to extract. However, this is debatable depending on the element being recovered, its value at the time and how energy-intensive the recovery process is.78,82,106 Cost is also a major factor in recycling on a cell level, where varying chemistries may dictate the separation process and thus the energy input and expenses, as well as the value of the final product.11,78 With this in mind, as suggested by the Öko-Institut, a revised EU Battery Directive may promote recycling on a cell level. The same process is already underway in China.76
A final hurdle in recycling, again with legislation, is the storage and transportation of lithium ion batteries. Undoubtedly, the cost of storage and transportation of lithium ion batteries is relatively high due to their classification as dangerous goods.107 Furthermore, there are stringent laws on how the battery is transported (i.e. contained or disassembled, via shipping or air), the state it is in (i.e. state of charge (SoC), any damage) and the purpose for transportation.108,109 This further complicates recycling if there is a need to transport batteries for sorting, disassembly, preparation for recycling (or reuse), recycling itself or storage to await commodity prices.82 This tends to suggest that future recycling should favour more localised recycling or pre-treatment plants, minimising transportation of spent units. Optimisation of the location of recycling plants relative to the waste streams that they serve, could aid in improving both the lifecycle impacts and techno-economics of this system. As mentioned above, there is a strong driver to co-locate manufacture and recycling and, given issue of logistics with the transport of batteries, these should be close to the vicinity of automobile manufacture.
Cogent arguments have been made for the decarbonisation of energy. A cornerstone of this is the decreased use of the internal combustion engine and a move to electrified transport, providing that the source of electricity is renewable. Lithium ion batteries are at present the favoured means of storing electrical power, but their ability to do this and not contribute to environmental waste depends on their ability to have a long service life and become part of a circular economy with sustainable, recyclable components.
Conclusion – design for recycle
This review has shown that many recycling issues originate from the design of the object and the strategy adopted for disassembly and recovery. The number of distinct phases in devices such as lithium ion batteries, coupled with their distribution in complex structures with inert separators, complicates the task of separation into individual pure phases. The current method of recycling lithium ion batteries uses pyrometallurgy, or comminution followed by hydrometallurgy. These methods lead to low value product streams or have a high cost and a low fraction of product recovery. Accordingly the saving accrued by using recycled material compared to never used material is in the range −5 to +20%.66,110At present lithium ion batteries are designed, built and traded without recycling in mind. With the inevitable increase in EV production over the coming years, it is vital that a scalable, suitable recycling scheme is implemented. Ideally, setting up a scheme whereby EoL batteries are returned to the manufacturers could spark the beginning of standardisation in which a lithium ion battery is designed for recycling.78
Development of appropriate legislation would incentivise manufacturers to recycle their EoL lithium ion batteries, as a closed-loop in a safe and economically viable manner. Resolving issues such as extended producer responsibility would certainly aid the creation of closed loop partnerships. It is clear that cell and pack design are vital to achieving an efficient and facile recycling process. It has recently been shown that separation of the electrode materials rather than shredding can lead to savings of up to 70% compared to never used material which is a compelling economic and environmental argument for separation.66 There are two large improvements that could be made in battery design to aid separation. The first is module-less packs with cells that are easy to separate and open. Automation of this process, particularly using robotics is an area of significant interest. The second area is the use of adhesives and binders which are important in both macroscopic disassembly (simplifying cell separation and opening) and microscopic electrode delamination (separating active material from current collectors). The development of reversible adhesives in electrode design or potentially even adhesiveless electrodes would simplify delamination and recovery of the active material. Improved pack labelling would also enable different battery chemistries to be separated before processing and reduce the risk of cross contamination.
An ideal battery for recycling would have a pack configuration with solid busbars in place of flexible cables, where large cells could be easily disassembled from the bulk structure. The cells could be autonomously dismantled and separated into anodes, cathodes and polymer separators. This would be assisted by slight changes in electrode and connector geometry without affecting electrode performance. The active materials on the electrodes could be delaminated and recovered if a binder was used which could be dispersed using aqueous solutions.
Design for recycle often involves minor changes to product structures but can help to establish a circular economy if it returns raw materials to the manufacturing process at a significantly reduced cost compared with primary sources. During a product's development there will be an initial phase where performance improvements are paramount and this is replaced by cost considerations as the product begins to attain a major market share. It is only at this point where environmental issues can influence product design. This is the point where lithium ion batteries are currently and concerted international activity is beginning to create a circular economy in technology metals which can truly influence the decarbonisation of transportation.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors would like to thank the Faraday Institution (grant codes FIRG005 and FIRG006, Project website https://relib.org.uk) for funding. LG also acknowledges the U.S. Department of Energy under contract DE-AC02-06CH11357 for funding. The authors would like to acknowledge the ReLiB research teams at Newcastle University and University of Birmingham for supplying the photographs used in Fig. 4. Thanks also to Hans-Eric Melin of Circular Energy Solutions for his dialogue with us around battery recycling rates.References
- U.S. Environmental Protection Agency, Facts and Figures about Materials, Waste and Recycling, https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/plastics-material-specific-data, (accessed 17 June 2020).
- J. L. Bower and C. M. Christensen, Disruptive Technologies: Catching the Wave, https://hbr.org/1995/01/disruptive-technologies-catching-the-wave, (accessed 23 June 2020).
- J. Horbach, C. Rammer and K. Rennings, Determinants of eco-innovations by type of environmental impact—The role of regulatory push/pull, technology push and market pull, Ecol. Econ., 2012, 78, 112–122 CrossRef.
- S. Deetman, S. Pauliuk, D. P. van Vuuren, E. van der Voet and A. Tukker, Scenarios for Demand Growth of Metals in Electricity Generation Technologies, Cars, and Electronic Appliances, Environ. Sci. Technol., 2018, 52, 4950–4959 CrossRef CAS.
- D. Linden and T. B. Reddy, Eds., in Handbook of batteries, McGraw-Hill, New York, 3rd edn, 2002, pp. 23.1–23.87 Search PubMed.
- G. J. May, A. Davidson and B. Monahov, Lead batteries for utility energy storage: A review, J. Energy Storage, 2018, 15, 145–157 CrossRef.
- European Conference of Ministers of Transport, Cutting Transport CO2 Emissions: What Progress?, Organisation for Economic Co-operation and Development, 2007 Search PubMed.
- K. Ogura and M. L. Kolhe, in Electric Vehicles: Prospects and Challenges, Elsevier, 2017, pp. 139–167 Search PubMed.
- L. Gaines, The future of automotive lithium-ion battery recycling: Charting a sustainable course, Sustainable Mater. Technol., 2014, 1–2, 2–7 CrossRef.
- H. E. Melin, Exploring the recycling rate of lithium-ion batteries, Circular Energy Storage, London, UK, 2020 Search PubMed.
- International Energy Agency, Global EV Outlook 2018, 2018, http://www.iea.org, (accessed 25/11/2018) Search PubMed.
- J. Lippert, Electric Vehicles, https://www.bloomberg.com/quicktake/electric-vehicles, (accessed 2 December 2019).
- L. C. Casals, B. A. García, F. Aguesse and A. Iturrondobeitia, Second life of electric vehicle batteries: relation between materials degradation and environmental impact, Int. J. Life Cycle Assess., 2017, 22, 82–93 CrossRef CAS.
- J. M. Turner and L. M. Nugent, Charging up Battery Recycling Policies: Extended Producer Responsibility for Single-Use Batteries in the European Union, Canada, and the United States: EPR for Single-Use Batteries, J. Ind. Ecol., 2016, 20, 1148–1158 CrossRef.
- Q. Dai, J. C. Kelly, L. Gaines and M. Wang, Life Cycle Analysis of Lithium-Ion Batteries for Automotive Applications, Batteries, 2019, 5, 48 CrossRef CAS.
- J. Marshall, D. Gastol, R. Sommerville, B. Middleton, V. Goodship and E. Kendrick, Disassembly of Li Ion Cells—Characterization and Safety Considerations of a Recycling Scheme, Metals, 2020, 10, 773 CrossRef.
- X. Zuo, J. Zhu, P. Müller-Buschbaum and Y.-J. Cheng, Silicon based lithium-ion battery anodes: A chronicle perspective review, Nano Energy, 2017, 31, 113–143 CrossRef CAS.
- C. P. Sandhya, B. John and C. Gouri, Lithium titanate as anode material for lithium-ion cells: a review, Ionics, 2014, 20, 601–620 CrossRef CAS.
- R. Schmuch, R. Wagner, G. Hörpel, T. Placke and M. Winter, Performance and cost of materials for lithium-based rechargeable automotive batteries, Nat. Energy, 2018, 3, 267–278 CrossRef CAS.
- N. Takami, K. Ise, Y. Harada, T. Iwasaki, T. Kishi and K. Hoshina, High-energy, fast-charging, long-life lithium-ion batteries using TiNb2O7 anodes for automotive applications, J. Power Sources, 2018, 396, 429–436 CrossRef CAS.
- H. Huo, Y. Xing, M. Pecht, B. J. Züger, N. Khare and A. Vezzini, Safety Requirements for Transportation of Lithium Batteries, Energies, 2017, 10, 793 CrossRef.
- Recommended Practices for Shipping Transport and Handling of Automotive-Type Battery System - Lithium Ion, https://www.sae.org/standards/content/j2950_202006/, (accessed 10 July 2020).
- L. Gaines and R. Cuenca, Costs of lithium-ion batteries for vehicles, 2000, http://www.osti.gov/servlets/purl/761281-9hcCH0/webviewable/, (accessed 23/06/2020) Search PubMed.
- S. Rothermel, M. Winter and S. Nowak, in Recycling of Lithium-Ion Batteries: The LithoRec Way, Springer Nature, Switzerland, 2018, pp. 1–33 Search PubMed.
- C. Helbig, A. M. Bradshaw, L. Wietschel, A. Thorenz and A. Tuma, Supply risks associated with lithium-ion battery materials, J. Clean. Prod., 2018, 172, 274–286 CrossRef CAS.
- X. Zheng, Z. Zhu, X. Lin, Y. Zhang, Y. He, H. Cao and Z. Sun, A Mini-Review on Metal Recycling from Spent Lithium Ion Batteries, Engineering, 2018, 4, 361–370 CrossRef CAS.
- H. Zheng, R. Yang, G. Liu, X. Song and V. S. Battaglia, Cooperation between Active Material, Polymeric Binder and Conductive Carbon Additive in Lithium Ion Battery Cathode, J. Phys. Chem. C, 2012, 116, 4875–4882 CrossRef CAS.
- S. S. Zhang, A review on electrolyte additives for lithium-ion batteries, J. Power Sources, 2006, 162, 1379–1394 CrossRef CAS.
- N. Nitta, F. Wu, J. T. Lee and G. Yushin, Li-ion battery materials: present and future, Mater. Today, 2015, 18, 252–264 CrossRef CAS.
- T. C. Nirmale, B. B. Kale and A. J. Varma, A review on cellulose and lignin based binders and electrodes: Small steps towards a sustainable lithium ion battery, Int. J. Biol. Macromol., 2017, 103, 1032–1043 CrossRef CAS.
- J. Warner, The Handbook of Lithium-Ion Battery Pack Design: Chemistry, Components, Types and Terminology, Elsevier, Amsterdam Boston Heidelberg, 2015 Search PubMed.
- G. Harper, R. Sommerville, E. Kendrick, L. Driscoll, P. Slater, R. Stolkin, A. Walton, P. Christensen, O. Heidrich, S. Lambert, A. Abbott, K. Ryder, L. Gaines and P. Anderson, Recycling lithium-ion batteries from electric vehicles, Nature, 2019, 575, 75–86 CrossRef CAS.
- R. Wang, L. Feng, W. Yang, Y. Zhang, Y. Zhang, W. Bai, B. Liu, W. Zhang, Y. Chuan, Z. Zheng and H. Guan, Effect of Different Binders on the Electrochemical Performance of Metal Oxide Anode for Lithium-Ion Batteries, Nanoscale Res. Lett., 2017, 12, 575 CrossRef.
- J. Li, Y. Lu, T. Yang, D. Ge, D. L. Wood and Z. Li, Water-Based Electrode Manufacturing and Direct Recycling of Lithium-Ion Battery Electrodes—A Green and Sustainable Manufacturing System, iScience, 2020, 23, 101081 CrossRef CAS.
- K. Wegener, S. Andrew, A. Raatz, K. Dröder and C. Herrmann, Disassembly of Electric Vehicle Batteries Using the Example of the Audi Q5 Hybrid System, Procedia CIRP, 2014, 23, 155–160 CrossRef.
- T. Waldmann, A. Iturrondobeitia, M. Kasper, N. Ghanbari, F. Aguesse, E. Bekaert, L. Daniel, S. Genies, I. J. Gordon, M. W. Löble, E. De Vito and M. Wohlfahrt-Mehrens, Review—Post-Mortem Analysis of Aged Lithium-Ion Batteries: Disassembly Methodology and Physico-Chemical Analysis Techniques, J. Electrochem. Soc., 2016, 163, A2149–A2164 CrossRef CAS.
- C. Herrmann, A. Raatz, M. Mennenga, J. Schmitt and S. Andrew, in Leveraging Technology for a Sustainable World, ed. D. A. Dornfeld and B. S. Linke, Springer Berlin Heidelberg, Berlin, Heidelberg, 2012, pp. 149–154 Search PubMed.
- C. Herrmann, A. Raatz, S. Andrew and J. Schmitt, Scenario-Based Development of Disassembly Systems for Automotive Lithium Ion Battery Systems, Adv. Mater. Res., 2014, 907, 391–401 Search PubMed.
- K. Wegener, W. H. Chen, F. Dietrich, K. Dröder and S. Kara, Robot Assisted Disassembly for the Recycling of Electric Vehicle Batteries, Procedia CIRP, 2015, 29, 716–721 CrossRef.
- V. K. Soo, Life Cycle Impact of Different Joining Decisions on Vehicle Recycling, The Australian National University, 2018, https://openresearch-repository.anu.edu.au/bitstream/1885/143902/1/Soo%20Thesis%202018.pdf, (accessed 23/06/2020) Search PubMed.
- A. Das, D. Li, D. Williams and D. Greenwood, Joining Technologies for Automotive Battery Systems Manufacturing, World Electr. Veh. J., 2018, 9, 22 CrossRef.
- L. Li, P. Zheng, T. Yang, R. Sturges, M. W. Ellis and Z. Li, Disassembly Automation for Recycling End-of-Life Lithium-Ion Pouch Cells, JOM, 2019, 71, 4457–4464 CrossRef CAS.
- BYD Company Ltd., BYD's New Blade Battery Set to Redefine EV Safety Standards, http://www.byd.com/en/news/2020-03-30/BYD%27s-New-Blade-Battery-Set-to-Redefine-EV-Safety-Standards, (accessed 19 June 2020).
- R. Sommerville, J. Shaw-Stewart, V. Goodship, N. Rowson and E. Kendrick, A review of physical processes used in the safe recycling of lithium ion batteries, Sustainable Mater. Technol., 2020, 25, e00197 CrossRef CAS.
- X. Wu, K. Song, X. Zhang, N. Hu, L. Li, W. Li, L. Zhang and H. Zhang, Safety Issues in Lithium Ion Batteries: Materials and Cell Design, Front. Energy Res., 2019, 7, 65 CrossRef.
- M. J. Lain, J. Brandon and E. Kendrick, Design Strategies for High Power vs. High Energy Lithium Ion Cells, Batteries, 2019, 5, 64 CrossRef CAS.
- M. V. Reddy, A. Mauger, C. M. Julien, A. Paolella and K. Zaghib, Brief History of Early Lithium-Battery Development, Materials, 2020, 13, 1884 CrossRef CAS.
- A. Yoshino, in Lithium-Ion Batteries, Elsevier, 2014, pp. 1–20 Search PubMed.
- R. Schröder, M. Aydemir and G. Seliger, Comparatively Assessing different Shapes of Lithium-ion Battery Cells, Procedia Manuf., 2017, 8, 104–111 CrossRef.
- X. Zhang, X. Chang, Y. Shen and Y. Xiang, Electrochemical-electrical-thermal modeling of a pouch-type lithium ion battery: An application to optimize temperature distribution, J. Energy Storage, 2017, 11, 249–257 CrossRef.
- Y. Zhao, L. B. Diaz, Y. Patel, T. Zhang and G. J. Offer, How to Cool Lithium Ion Batteries: Optimising Cell Design using a Thermally Coupled Model, J. Electrochem. Soc., 2019, 166, A2849–A2859 CrossRef CAS.
- K. Liu, Y. Liu, D. Lin, A. Pei and Y. Cui, Materials for lithium-ion battery safety, Sci. Adv., 2018, 4, eaas9820 CrossRef.
- T. Song, Y. Li, J. Song and Z. Zhang, Airworthiness Considerations of Supply Chain Management from Boeing 787 Dreamliner Battery Issue, Procedia Eng., 2014, 80, 628–637 CrossRef.
- S. Sloop, L. Crandon, M. Allen, K. Koetje, L. Reed, L. Gaines, W. Sirisaksoontorn and M. Lerner, A direct recycling case study from a lithium-ion battery recall, Sustainable Mater. Technol., 2020, 25, e00152 CrossRef CAS.
- T. R. Beatty, Battery assembly, US4049882A, 1977, 1–7 Search PubMed.
- M. Müller, L. Pfaffmann, S. Jaiser, M. Baunach, V. Trouillet, F. Scheiba, P. Scharfer, W. Schabel and W. Bauer, Investigation of binder distribution in graphite anodes for lithium-ion batteries, J. Power Sources, 2017, 340, 1–5 CrossRef.
- Y. Ma, J. Ma and G. Cui, Small things make big deal: Powerful binders of lithium batteries and post-lithium batteries, Energy Storage Mater., 2019, 20, 146–175 CrossRef.
- J.-T. Li, Z.-Y. Wu, Y.-Q. Lu, Y. Zhou, Q.-S. Huang, L. Huang and S.-G. Sun, Water Soluble Binder, an Electrochemical Performance Booster for Electrode Materials with High Energy Density, Adv. Energy Mater., 2017, 7, 1701185 CrossRef.
- S. Lim, S. Kim, K. H. Ahn and S. J. Lee, The effect of binders on the rheological properties and the microstructure formation of lithium-ion battery anode slurries, J. Power Sources, 2015, 299, 221–230 CrossRef CAS.
- D. E. Demirocak and B. Bhushan, Probing the aging effects on nanomechanical properties of a LiFePO4 cathode in a large format prismatic cell, J. Power Sources, 2015, 280, 256–262 CrossRef CAS.
- J. S. Terreblanche, D. L. Thompson, I. M. Aldous, J. Hartley, A. P. Abbott and K. S. Ryder, Experimental Visualization of Commercial Lithium Ion Battery Cathodes: Distinguishing Between the Microstructure Components Using Atomic Force Microscopy, J. Phys. Chem. C, 2020, 124, 14622–14631 CrossRef CAS.
- M. M. Kabir and D. E. Demirocak, Degradation mechanisms in Li-ion batteries: a state-of-the-art review: Degradation Mechanisms in Li-ion Batteries: A State-of-the-Art Review, Int. J. Energy Res., 2017, 41, 1963–1986 CrossRef CAS.
- D. Bresser, D. Buchholz, A. Moretti, A. Varzi and S. Passerini, Alternative binders for sustainable electrochemical energy storage – the transition to aqueous electrode processing and bio-derived polymers, Energy Environ. Sci., 2018, 11, 3096–3127 RSC.
- X. Zeng, J. Li and N. Singh, Recycling of Spent Lithium-Ion Battery: A Critical Review, Crit. Rev. Environ. Sci. Technol., 2014, 44, 1129–1165 CrossRef CAS.
- W. Lv, Z. Wang, H. Cao, Y. Sun, Y. Zhang and Z. Sun, A Critical Review and Analysis on the Recycling of Spent Lithium-Ion Batteries, ACS Sustainable Chem. Eng., 2018, 6, 1504–1521 CrossRef CAS.
- D. Thompson, C. Hyde, J. M. Hartley, A. P. Abbott, P. Anderson and G. D. J. Harper, To shred or not to shred: Techno-economic assessment of Lithium ion battery hydrometallurgical recycling, Resour., Conserv. Recycl., 2020 Search PubMed, submitted.
- J. Li, G. Wang and Z. Xu, Generation and detection of metal ions and volatile organic compounds (VOCs) emissions from the pretreatment processes for recycling spent lithium-ion batteries, Waste Manage., 2016, 52, 221–227 CrossRef CAS.
- D. Hauk and M. Kurrat, in Recycling of Lithium-Ion Batteries: The LithoRec Way, Springer Nature, Switzerland, 2018, pp. 53–81 Search PubMed.
- S. Al-Thyabat, T. Nakamura, E. Shibata and A. Iizuka, Adaptation of minerals processing operations for lithium-ion (LiBs) and nickel metal hydride (NiMH) batteries recycling: Critical review, Miner. Eng., 2013, 45, 4–17 CrossRef CAS.
- C. Ekberg and M. Petranikova, in Lithium Process Chemistry, Elsevier, 2015, pp. 233–267 Search PubMed.
- F. Larouche, F. Tedjar, K. Amouzegar, G. Houlachi, P. Bouchard, G. P. Demopoulos and K. Zaghib, Progress and Status of Hydrometallurgical and Direct Recycling of Li-Ion Batteries and Beyond, Materials, 2020, 13, 801 CrossRef CAS.
- M. Grützke, V. Kraft, B. Hoffmann, S. Klamor, J. Diekmann, A. Kwade, M. Winter and S. Nowak, Aging investigations of a lithium-ion battery electrolyte from a field-tested hybrid electric vehicle, J. Power Sources, 2015, 273, 83–88 CrossRef.
- A. Chagnes, in Lithium Process Chemistry, Elsevier, 2015, pp. 41–80 Search PubMed.
- J. B. Goodenough and Y. Kim, Challenges for Rechargeable Li Batteries, Chem. Mater., 2010, 22, 587–603 CrossRef CAS.
- E. A. Olivetti, G. Ceder, G. G. Gaustad and X. Fu, Lithium-Ion Battery Supply Chain Considerations: Analysis of Potential Bottlenecks in Critical Metals, Joule, 2017, 1, 229–243 CrossRef.
- K. Meyer, M. Buchert, S. Degreif and P. Dolega, Ensuring a Sustainable Supply of Raw Materials for Electric Vehicles, Öko-Institut, 2018, https://www.agora-verkehrswende.de/fileadmin/Projekte/2017/Nachhaltige_Rohstoffversorgung_Elektromobilitaet/Agora_Verkehrswende_Rohstoffestrategien_EN_WEB.pdf, (accessed 21/11/2018) Search PubMed.
- T. P. Narins, The battery business: Lithium availability and the growth of the global electric car industry, Extr. Ind. Soc., 2017, 4, 321–328 Search PubMed.
- L. Yun, D. Linh, L. Shui, X. Peng, A. Garg, M. L. P. Le, S. Asghari and J. Sandoval, Metallurgical and mechanical methods for recycling of lithium-ion battery pack for electric vehicles, Resour., Conserv. Recycl., 2018, 136, 198–208 CrossRef.
- O. Velázquez-Martínez, J. Valio, A. Santasalo-Aarnio, M. Reuter and R. A. Serna-Guerrero, A Critical Review of Lithium-Ion Battery Recycling Processes from a Circular Economy Perspective, Batteries, 2019, 5, 68 CrossRef.
- X. Wang, G. Gaustad, C. W. Babbitt and K. Richa, Economies of scale for future lithium-ion battery recycling infrastructure, Resour., Conserv. Recycl., 2014, 83, 53–62 CrossRef.
- BRUNP: New 100,000-ton LIB recycling plant in Hunan Province, https://www.iru-miru.com/en/article_detail.php?id=29876, (accessed 23 June 2020).
- H. E. Melin, The Lithium-ion Battery End-of-Life market 2018–2025, Circular Energy Storage, 2018 Search PubMed.
- J. B. Dunn, L. Gaines, M. Barnes, J. L. Sullivan and M. Wang, Material and Energy Flows in the Materials Production, Assembly, and End-of-Life Stages of the Automotive Lithium-Ion Battery Life Cycle, Argonne National Laboratory, 2014 Search PubMed.
- J. Diekmann, C. Hanisch, L. Froböse, G. Schälicke, T. Loellhoeffel, A.-S. Fölster and A. Kwade, Ecological Recycling of Lithium-Ion Batteries from Electric Vehicles with Focus on Mechanical Processes, J. Electrochem. Soc., 2017, 164, 6184–6191 CrossRef.
- B. Huang, Z. Pan, X. Su and L. An, Recycling of lithium-ion batteries: Recent advances and perspectives, J. Power Sources, 2018, 399, 274–286 CrossRef CAS.
- Electric Vehicles: Driving the Transition, House of Commons, 2018, https://publications.parliament.uk/pa/cm201719/cmselect/cmbeis/383/383.pdf, (accessed 29/03/2019).
- A. Charlish and Z. Shabalala, Belgium's Umicore plans to ramp up EV battery recycling capacity: CEO, https://uk.reuters.com/article/us-umicore-recycling/belgiums-umicore-plans-to-ramp-up-ev-battery-recycling-capacity-ceo-idUKKBN1KN1ZO, (accessed 29 March 2019).
- T. Parry, Vehicle Licensing Statistics: Quarter 3 (Jul–Sep) 2018, HM Government, 2018, https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/763792/vehicle-licensing-statistics-july-to-september.pdf Search PubMed, (accessed 29/03/2019).
- G. Williams, ELVs: Carrot or stick?, CIWM, 2017, pp. 1–3, http://uk.emrgroup.com/files/ciwm-may-2017_emr.pdf, (accessed 29/03/2019) Search PubMed.
- T. Zenger, A. Krebs and H. J. Hendrik van Deutekom, Method of and Apparatus for Dismantling and Storage of Objects Comprising Alkali Metals, such as Alkali Metal Containing Batteries, US007833646B2, 2010, 1–11 Search PubMed.
- Y. Shi, G. Chen and Z. Chen, Effective regeneration of LiCoO 2 from spent lithium-ion batteries: a direct approach towards high-performance active particles, Green Chem., 2018, 20, 851–862 RSC.
- S. Kim, D. Yang, K. Rhee and J. Sohn, Recycling process of spent battery modules in used hybrid electric vehicles using physical/chemical treatments, Res. Chem. Intermed., 2014, 40, 2447–2456 CrossRef CAS.
- D. Dutta, A. Kumari, R. Panda, S. Jha, D. Gupta, S. Goel and M. K. Jha, Close loop separation process for the recovery of Co, Cu, Mn, Fe and Li from spent lithium-ion batteries, Sep. Purif. Technol., 2018, 200, 327–334 CrossRef CAS.
- W. Gao, X. Zhang, X. Zheng, X. Lin, H. Cao, Y. Zhang and Z. Sun, Lithium Carbonate Recovery from Cathode Scrap of Spent Lithium-Ion Battery: A Closed-Loop Process, Environ. Sci. Technol., 2017, 51, 1662–1669 CrossRef CAS.
- Y. Huang, G. Han, J. Liu, W. Chai, W. Wang, S. Yang and S. Su, A stepwise recovery of metals from hybrid cathodes of spent Li-ion batteries with leaching-flotation-precipitation process, J. Power Sources, 2016, 325, 555–564 CrossRef CAS.
- G. Prabaharan, S. P. Barik, N. Kumar and L. Kumar, Electrochemical process for electrode material of spent lithium ion batteries, Waste Manage., 2017, 68, 527–533 CrossRef CAS.
- J. Hu, J. Zhang, H. Li, Y. Chen and C. Wang, A promising approach for the recovery of high value-added metals from spent lithium-ion batteries, J. Power Sources, 2017, 351, 192–199 CrossRef CAS.
- Y. Yang, S. Xu and Y. He, Lithium recycling and cathode material regeneration from acid leach liquor of spent lithium-ion battery via facile co-extraction and co-precipitation processes, Waste Manage., 2017, 64, 219–227 CrossRef CAS.
- S. P. Barik, G. Prabaharan and L. Kumar, Leaching and separation of Co and Mn from electrode materials of spent lithium-ion batteries using hydrochloric acid: Laboratory and pilot scale study, J. Clean. Prod., 2017, 147, 37–43 CrossRef CAS.
- L.-P. He, S.-Y. Sun, X.-F. Song and J.-G. Yu, Leaching process for recovering valuable metals from the LiNi 1/3 Co 1/3 Mn 1/3 O 2 cathode of lithium-ion batteries, Waste Manage., 2017, 64, 171–181 CrossRef CAS.
- W. Xuan, A. Otsuki and A. Chagnes, Investigation of the leaching mechanism of NMC 811 (LiNi0.8Mn0.1Co0.1O2) by hydrochloric acid for recycling lithium ion battery cathodes, RSC Adv., 2019, 9, 38612–38618 RSC.
- Umicore - Our Recycling Process, https://csm.umicore.com/en/recycling/battery-recycling/our-recycling-process, (accessed 4 April 2019).
- N. P. Lebedeva and L. Boon-Brett, Considerations on the Chemical Toxicity of Contemporary Li-Ion Battery Electrolytes and Their Components, J. Electrochem. Soc., 2016, 163, A821–A830 CrossRef CAS.
- O. Clemens and P. R. Slater, Topochemical modifications of mixed metal oxide compounds by low-temperature fluorination routes, Rev. Inorg. Chem., 2013, 33, 105–117 CAS.
- A. M. Haregewoin, A. S. Wotango and B.-J. Hwang, Electrolyte additives for lithium ion battery electrodes: progress and perspectives, Energy Environ. Sci., 2016, 9, 1955–1988 RSC.
- D. J. Tytgat, The Recycling Efficiency of Li-ion EV batteries according to the European Commission Regulation, and the relation with the End-of-Life Vehicles Directive recycling rate, World Electr. Veh. J., 2013, 6, 1039–1047 CrossRef.
- Department for Transport, Transporting hybrid lithium batteries, HM Government, 2016, https://www.gov.uk/government/publications/carriage-of-dangerous-goods-multilateral-agreement-adr-m296, (accessed 16/05/2019) Search PubMed.
- International Air Transport Association, Lithium Batteries, https://www.iata.org/whatwedo/cargo/dgr/Pages/lithium-batteries.aspx, (accessed 16 May 2019).
- DHL, Shipping Lithium Batteries, https://www.dhl.co.uk/en/express/shipping/shipping_advice/lithium_batteries.html, (accessed 16 May 2019).
- A. Sattar, D. Greenwood, M. Dowson and P. Unadkat, Automotive Lithium ion Battery Recycling in the UK, WMG, The University of Warwick, 2020, https://warwick.ac.uk/fac/sci/wmg/business/transportelec/22350m_wmg_battery_recycling_report_v7.pdf, (accessed 05/10/2020) Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0gc02745f |
| This journal is © The Royal Society of Chemistry 2020 |