Themed collection New Directions in Natural Product Synthesis

New directions in natural product synthesis
Guest Editors Huw M. L. Davies, Kenichiro Itami and Brian M. Stoltz introduce this themed issue on natural product synthesis.

Chem. Soc. Rev., 2018,47, 7828-7829
https://doi.org/10.1039/C8CS90115E
Recent applications of C–H functionalization in complex natural product synthesis
In this review, recent examples featuring C–H functionalization in the synthesis of complex natural products are discussed.
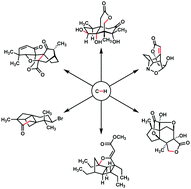
Chem. Soc. Rev., 2018,47, 8925-8967
https://doi.org/10.1039/C8CS00716K
Application of (4+3) cycloaddition strategies in the synthesis of natural products
This review summarizes the applications of (4+3) cycloadditions, both classical and formal, in the syntheses of natural products in the last two decades.
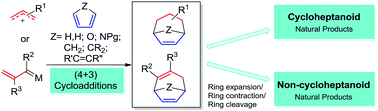
Chem. Soc. Rev., 2018,47, 8881-8924
https://doi.org/10.1039/C8CS00532J
Aryne-based strategy in the total synthesis of naturally occurring polycyclic compounds
This review has outlined the strategies and tactics of using arynes in the total syntheses of polycyclic natural products.
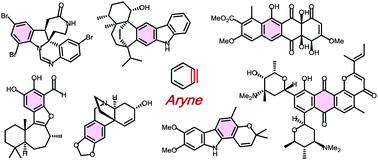
Chem. Soc. Rev., 2018,47, 8030-8056
https://doi.org/10.1039/C8CS00350E
Oxidative coupling strategies for the synthesis of indole alkaloids
Direct functionalization of indole through oxidative coupling reactions with enolates or phenols provides a powerful tool for assembling indole alkaloids.

Chem. Soc. Rev., 2018,47, 8018-8029
https://doi.org/10.1039/C8CS00305J
Amide activation: an emerging tool for chemoselective synthesis
This review focusses on the use of amide activation for chemoselective functionalisation and its application in natural product synthesis.

Chem. Soc. Rev., 2018,47, 7899-7925
https://doi.org/10.1039/C8CS00335A
Recent advances in chemical dearomatization of nonactivated arenes
A comprehensive review of recent developments and applications of dearomatization of simple, nonactivated aromatic compounds.
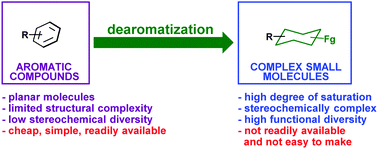
Chem. Soc. Rev., 2018,47, 7996-8017
https://doi.org/10.1039/C8CS00389K
Enabling strategies for step efficient syntheses
The field of natural product total synthesis has reached the point where synthetic efficiency has become more important than merely defining a viable (yet less ideal) route to the target molecule. Several synthesis of different types of natural products are compared using color-coded flow charts.

Chem. Soc. Rev., 2018,47, 7985-7995
https://doi.org/10.1039/C8CS00399H
Gold-catalyzed glycosylation in the synthesis of complex carbohydrate-containing natural products
Gold(I)- and gold(III)-catalyzed glycosylation reactions and their application in the synthesis of natural glycoconjugates are reviewed.
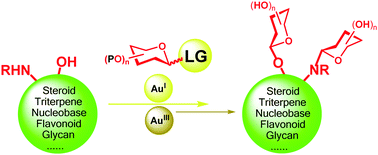
Chem. Soc. Rev., 2018,47, 7954-7984
https://doi.org/10.1039/C8CS00209F
Recent advances in the application of Diels–Alder reactions involving o-quinodimethanes, aza-o-quinone methides and o-quinone methides in natural product total synthesis
This review summarizes recent advances in Diels–Alder reactions involving o-QDMs, o-QMs and aza-o-QMs. The power and potential of this strategy in organic synthesis and natural product total synthesis is highlighted.
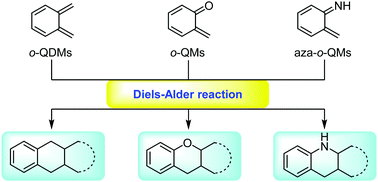
Chem. Soc. Rev., 2018,47, 7926-7953
https://doi.org/10.1039/C8CS00274F
Beyond olefins: new metathesis directions for synthesis
This tutorial review provides an introduction to metathesis reactions between carbonyls and olefins or alkynes and their application in natural product synthesis.
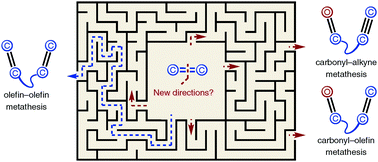
Chem. Soc. Rev., 2018,47, 7867-7881
https://doi.org/10.1039/C8CS00391B
Metamorphosis of cycloalkenes for the divergent total synthesis of polycyclic indole alkaloids
This review summarizes the divergent synthesis of monoterpene indole alkaloids using cycloalkene as the turning point of structural diversity.
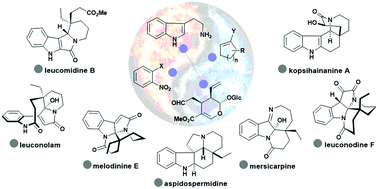
Chem. Soc. Rev., 2018,47, 7882-7898
https://doi.org/10.1039/C8CS00454D
Radicals in natural product synthesis
Free radical intermediates have intrigued chemists since their discovery, and an ever-increasing appreciation for their unique reactivity has resulted in the widespread utilization of these species for natural product synthesis.
Chem. Soc. Rev., 2018,47, 7851-7866
https://doi.org/10.1039/C8CS00379C
Computational chemistry strategies in natural product synthesis
Computational chemistry has made profound contributions to natural product synthesis, as showcased in several impressive examples.
Chem. Soc. Rev., 2018,47, 7830-7844
https://doi.org/10.1039/C8CS00351C
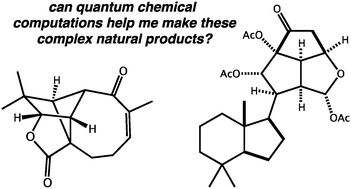
Questions in natural products synthesis research that can (and cannot) be answered using computational chemistry
Questions of relevance to synthetic chemists that can be answered, at least in part, using quantum chemical computations are highlighted.
Chem. Soc. Rev., 2018,47, 7845-7850
https://doi.org/10.1039/C8CS00298C
About this collection
We are delighted to present a Chemical Society Reviews themed collection on ‘New enabling strategies for natural products synthesis’. This issue, guest edited by Brian Stoltz (California Institute of Technology), Ken Itami (Nagoya University) and Huw M. L. Davies (Emory University), focusses on new enabling methodologies and their applications to the synthesis of complex natural products. The collection aims to highlight the major advances that have been made to streamline the synthesis of complex targets, as well as showcase the high level of innovation that drives complex synthesis and the value of natural product applications.