Electrosynthesis of functionalized tetrahydrocarbazoles via sulfonylation triggered cyclization reaction of indole derivatives†
Abstract
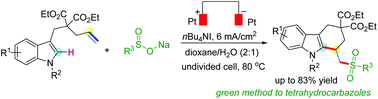
Synthesis of tetrahydrocarbazoles is of great interest due to its importance in organic and biological chemistry. A method for access to such heterocyclic compounds usually needs harsh reaction conditions or transition-metal catalysts. Herein, we developed an efficient reaction of easily available indole derivatives and sodium sulfinates to achieve functionalized tetrahydrocarbazoles under electrochemical conditions. This reaction proceeds through the sequence of sulfonylation, cycloaddition and deprotonation, and tolerated the two coupling partners with a variety of electronically and sterically diverse substituents. This electrochemical method obviates the use of any transition-metal catalyst and chemical oxidizing reagent, which provides an efficient and green strategy to prepare sulfonylalkyl substituted tetrahydrocarbazoles.


 Please wait while we load your content...
Please wait while we load your content...