Chemo- and regioselective nucleophilic hydrofunctionalization of unactivated aliphatic alkenes under transition-metal-free catalysts†
Abstract
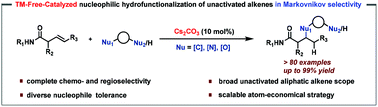
Transition-metal-free-catalyzed nucleophilic hydrofunctionalization of both terminal and internal unactivated aliphatic alkenes has been described for the first time. Most topical classes of carbon, nitrogen and oxygen nucleophiles are well-compatible. The highly chemoselective unprotected dinucleophiles are also presented in the atom-economical approach. More than 80 structurally complex β-hetero-substituted aliphatic amides were rapidly synthesized in good yield with exclusive Markovnikov selectivity, which are difficult to be achieved efficiently by the traditional Michael addition of conjugated amides due to their poor intrinsic electrophilicity.


 Please wait while we load your content...
Please wait while we load your content...