An environmentally responsible route to tezacaftor, a drug for treatment of cystic fibrosis prepared in water via ppm Au catalysis as entry to 2-substituted indoles†
Abstract
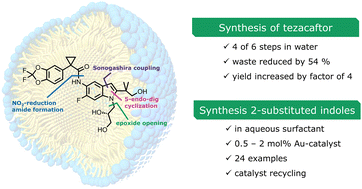
An environmentally responsible synthesis of tezacaftor, a drug approved in 2018 currently in use for treatment of cystic fibrosis, is described utilizing chemistry in water enabled by the nonionic surfactant TPGS-750-M. The route relies on a newly developed ppm level Au-catalyzed 5-endo-dig cyclization of ortho-acetylenic anilines to form the required indole moiety as a key step. Several examples showcase the generality of this cyclization. Assessment of the greenness of this approach is made by comparison of the overall E Factor, which is reduced by 54% relative to the existing route to tezacaftor. DFT analysis rationalizes the important role played by micellar nanoreactors, as well as indicating the location of the recyclable catalyst within the aqueous reaction medium.


 Please wait while we load your content...
Please wait while we load your content...