Photoredox-catalyzed regio- & stereoselective C(sp2)–H cyanoalkylation of enamides with cycloketone oximes via selective C–C bond cleavage/radical addition cascade†
Abstract
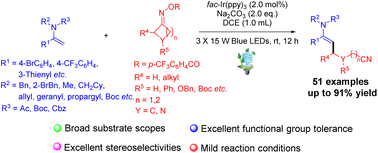
A photoredox-catalyzed regio- and stereoselective Heck-type cyanoalkylation of synthetically prominent enamides with cycloketone oximes via selective β-C–C bond scission/selective radical addition cascade is developed, enabling the incorporation of synthetically versatile and pharmaceutically appealing distal cyanoalkyl moieties into enamide scaffolds under mild conditions. The synthetic importance of this methodology was highlighted by the broad substrate scopes, satisfying functional group compatibilities, excellent regio- and stereoselectivities as well as the versatile and diverse synthetic applications of β-cyanoalkylated enamides.


 Please wait while we load your content...
Please wait while we load your content...