Interpreting the variations in the kinetic and potential energies in the formation of a covalent bond
Abstract
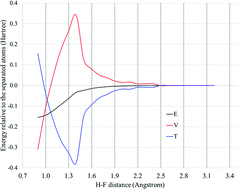
We address the long-standing controversy as to the physical origin of covalent bonding, whether it involves a lowering of the potential energy or a lowering of the kinetic energy. We conclude that both of these do occur and contribute to the formation of the bond. The analysis is in terms of the virial theorem and the variations in the potential energy and the kinetic energy as the atoms approach each other. At large separations, the change in kinetic energy relative to the separated atoms is negative and stabilizing, while the corresponding potential energy change is positive and destabilizing. However, as the atoms approach their equilibrium separation, these rapidly reverse; the kinetic energy increases and the potential energy decreases, so that at equilibrium the net kinetic energy is positive and the net potential energy negative. At equilibrium, the bonding is due solely to the potential energy and is electrostatic.
- This article is part of the themed collection: PCCP Reviews


 Please wait while we load your content...
Please wait while we load your content...