Stepwise dissociation of ion pairs by water molecules: cation-dependent separation mechanisms between carboxylate and alkali-earth metal ions†
Abstract
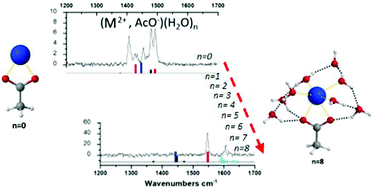
Microhydrated H2-tagged ion pairs (Ca2+, AcO−)(H2O)n=0–8 and (Ba2+, AcO−)(H2O)n=0–5 are investigated by IR photodissociation laser spectroscopy and DFT-D frequency calculations. The detailed picture of the first steps of ion dissociation reveals two mechanisms, where water molecules promote dissociation either directly or indirectly depending on the nature of the cation.
- This article is part of the themed collection: 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...