Enhancement of propene oligomerization and aromatization by proximate protons in zeolites; FTIR study of the reaction pathway in ZSM-5†
Abstract
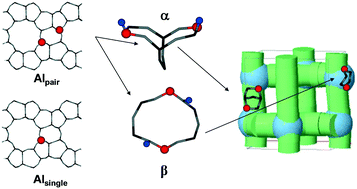
The enhanced effect of strongly acidic proximate protons (distance 5.0–5.5 Å) in ZSM-5 was presented on complex propene oligomerization up to the aromatization and development of individual carbenium ion intermediates in the zeolite pores. H-ZSM-5 samples were hydrothermally synthesized in order to possess similar contents of framework Al (Si/Al 23.8 and 24.5) located at channel intersections; they also had greatly predominated population of proximate protons defined by their bonding to two AlO4− ions located in one 6MR or possessed big fraction of far distant protons attached to single AlO4− in different rings. The locations of the Al(H+) sites were obtained from the analysis of 27Al (3Q) and 29Si MAS NMR and Co(II) ion-exchange and d–d transitions of bare Co(II) ions. The turnover rates for the conversion of propene to C4–C9 olefins were 5–8 times higher and that to BTX aromatics were up to 20 times higher over proximate Brønsted protons compared with that of single far distant sites. The reaction progress monitored by in situ time-resolved FTIR spectroscopy showed the step-wise formation of saturated (Cn+), alkenyl (Cnm=+) and aromatic carbenium ions formed by olefins' protonation and intermolecular hydride ion transfers, respectively, developing faster over the proximate protons. The similar apparent activation energies and enthalpies for propene conversion over the proximate and single protons but significantly less negative apparent entropy found with proximate sites suggested a later transition state closer to products. Both the polarization of reactants and steric constraints for the carbenium ion intermediates bound on the proximate sites, causing less freedom in a transition state and faster deprotonation of carbenium ions, were suggested to contribute to the enhanced reaction rate.
- This article is part of the themed collection: 2019 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...