Development of MgCo2O4–BaCO3 composites as microwave catalysts for the highly effective direct decomposition of NO under excess O2 at a low temperature†
Abstract
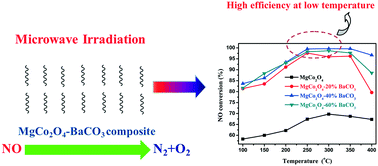
The catalytic NO decomposition reaction is a hot research topic. However, it is still a huge challenge to achieve the NO decomposition reaction with high efficiency under excess O2 at a low temperature. Herein, we developed MgCo2O4–BaCO3 composites as novel microwave catalysts for the highly effective direct decomposition of NO by microwave catalysis under excess O2 at a low temperature. Importantly, impressively high NO conversion of 99.6% and N2 selectivity of 97.8% could be achieved over MgCo2O4–40%BaCO3 at 250 °C under excess O2; this NO conversion value was much higher than the highest NO conversion value reported over single MgCo2O4 (only 69.7%). Comparatively, the highest NO conversion and N2 yield were only 29.5% and 15.1%, respectively, for MgCo2O4–40%BaCO3 at 650 °C in the conventional reaction mode under identical conditions. O2-TPD and XPS analyses of the catalysts were performed to demonstrate the possible reasons for such differences in catalytic activities. The apparent activation energies of MgCo2O4, MgCo2O4–20%BaCO3, MgCo2O4–40%BaCO3, and MgCo2O4–60%BaCO3 were as low as 45.7, 26.4, 22.1, and 27.5 kJ mol−1, respectively, indicating a significant microwave catalytic effect. In this work, we developed an attractive avenue for highly effective and environmentally friendly NOx emission control and provided some guidelines for the rational design of outstanding microwave catalysts in microwave chemistry.


 Please wait while we load your content...
Please wait while we load your content...