The Ugi three-component reaction and its variants
Abstract
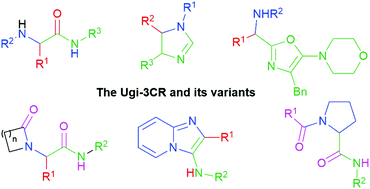
More than 60 years have passed since I. Ugi synthesized the first α-amidoamides in one experimental step involving a combination of aldehydes, amines, carboxylic acids and isocyanides, a multicomponent reaction (MCR) known later as the Ugi four-component reaction (U-4CR), in honor of the pioneer of the modern MCR-based organic synthesis. Then, a broad variety of valued molecules (α-aminoamides) in various fields of science and technology have been synthesized via a three-component version of the Ugi reaction (U-3CR or truncated Ugi reaction), which makes use of a carbonylic component (aldehydes or ketones), amines and isocyanides. Moreover, some variants of the U-3CR have been developed, including its combinations with further processes such as functionalizations, condensations, cross couplings, cyclizations, ring openings, rearrangements, and so on. Also, some robust computational calculations for understanding the energy profiles of Ugi-type 3CR have been performed. All these topics are in the scope of the present review, which covers selected works mainly from the last decade, focusing on relevant contributions to heterocyclic chemistry, and especially on novel and complex reaction mechanisms. The manuscript has been categorized by the name/type reaction approach, aiming to give readers novel insights for further investigations.
- This article is part of the themed collections: Celebrating Latin American Chemistry and 2021 Organic Chemistry Frontiers Review-type Articles


 Please wait while we load your content...
Please wait while we load your content...