Experimental lipophilicity scale for coded and noncoded amino acid residues†
Abstract
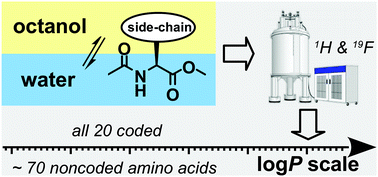
Among other features, the polarity of amino acid residues is the key parameter for understanding their role in proteins. The wide occurrence of protein modifications in nature and the advent of genetic code engineering techniques created a need for an experimental polarity value integrating both coded (canonical) and noncoded (noncanonical) residues on one universal scale. To address this issue, this work reports on a polarity scale based on the experimental lipophilicity of methyl esters of N-acetylamino acids. The derivatization of amino acids was performed in two steps under mild conditions that allowed conversion of a wide array of amino acids into analytical derivatives. The partitioning/distribution between octan-1-ol and water/buffer was measured using the intensity of the NMR signal as a characteristic for the concentration. The reference set of twenty coded amino acids generated log P values spanning 5.1 units: from tryptophan being the most hydrophobic to aspartate being the most hydrophilic. Furthermore, lipophilicity was measured for a set of analogues of phenylalanine, tyrosine, tryptophan, methionine, proline, and lysine that are typical in nature and/or laboratory practice. The polarity scale reported here will aid the rationalization of amino acid replacements in proteins, and will guide further efforts in experimental genetic code engineering.
- This article is part of the themed collections: Chemical Biology in OBC and Mechanistic, computational & physical organic chemistry in OBC

 Please wait while we load your content...
Please wait while we load your content...