Metal-free synthesis of quinoline-2,4-dicarboxylate derivatives using aryl amines and acetylenedicarboxylates through a pseudo three-component reaction†
Abstract
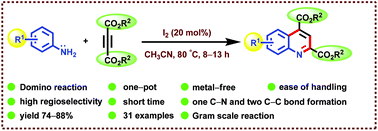
An efficient, useful and one-pot protocol for the synthesis of quinoline-2,4-dicarboxylate scaffolds is accomplished from aryl amines and dimethyl/diethyl acetylenedicarboxylates using 20 mol% molecular iodine as a catalyst in acetonitrile at 80 °C. In addition, the mechanistic explanation for the formation of the desired products is disclosed. The pivotal role of molecular iodine in the formation of the major products, diester quinoline derivatives, and the minor product, triesters, in two cases is described in the mechanism. The notable advantages of this method are non-involvement of a metal catalyst, avoiding of metal contamination in the final product as well as waste generation, use of a low cost and eco-friendly catalyst, ease of handling, high regioselectivity, shorter reaction time, the formation of one C–N and two C–C bonds and a broad substrate scope with good yields.
- This article is part of the themed collection: Synthetic methodology in OBC

 Please wait while we load your content...
Please wait while we load your content...