Triethylamine-methanol mediated selective removal of oxophenylacetyl ester in saccharides†
Abstract
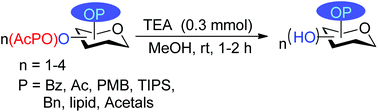
A highly selective, mild, and efficient method for the cleavage of oxophenylacetyl ester protected saccharides was developed using triethylamine in methanol at room temperature. The reagent proved successful against different labile groups like acetal, ketal, and PMB and also generated good yields of the desired saccharides bearing lipid esters. Further, we also observed DBU in methanol as an alternative reagent for the deprotection of acetyl, benzoyl, and oxophenylacetyl ester groups.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...