The structural and magnetic properties of BaFe12O19 nanoparticles: effect of residual sodium ions†
Abstract
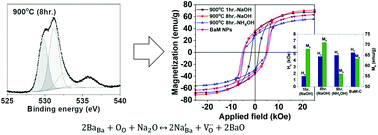
We studied the structural and magnetic properties of BaFe12O19 (BaM) nanoparticles synthesized by a co-precipitation route based on a modified citrate process. The lattice contraction of co-precipitated BaM nanoparticles is detected after prolonged sintering at 900 °C. In addition, investigation with X-ray photoelectron spectroscopy (XPS) provides evidence of a highly enhanced population of oxygen vacancies. The lattice distortion and formation of oxygen vacancies are attributed to a direct result of substitutional incorporation of smaller Na ions into Ba2+ sites in the lattice of BaM during prolonged annealing. The aliovalent impurities are assumed to be originated from NaOH which has been incorporated as a pH modifier. Magnon scattering is detected at 1640 cm−1 in the low temperature Raman spectra of BaM. Minimization of the magnon peak is also identified after prolonged annealing, which indicates oxygen vacancy-induced collapse of strong anti-ferromagnetic interaction between Fe3+ ions in the bipyramidal sites and the octahedral sites in BaM nanoparticles. As a result, the saturation magnetization (Ms) of BaM nanoparticles is enhanced by the onset of local ferromagnetic interaction induced by the collapse of antiferromagnetic interaction between oppositely aligned spins. In this study, we re-investigated the evolution of structural and magnetic properties with prolonged annealing of BaM nanoparticles and the effects of residual Na ions are also discussed.


 Please wait while we load your content...
Please wait while we load your content...