Recent developments in the synthesis and applications of furopyrazoles
Abstract
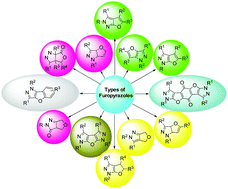
This review aims to describe the different strategies developed so far for the synthesis of furo[2,3-c]pyrazole, furo[3,2-c]pyrazole, dihydrofuro[2,3-c]pyrazole, dihydrofuro[3,4-c]pyrazole tetrahydrofuro[3,4-c]pyrazole, tetrahydrofuro[3,2-c]pyrazole, hexahydrofuro[3,4-c]pyrazole, furo-di-pyrazole and bis-furopyrazole derivatives and their biological and pharmacological properties such as antiallergic effects, anticancer, antibacterial, antifungal, antimicrobial, and anti-leukemia activities, high affinity to CB2 receptors, high σ1 receptor affinity and antioxidant activities. Owing to this diversity in the biological field, they have attracted the attention of many researchers to study their synthetic methods and biological activities. Our research efforts have been focusing on different aspects of furopyrazole derivatives including facile synthesis and investigating their bioactivity.
- This article is part of the themed collection: 2020 Focus and Perspective articles


 Please wait while we load your content...
Please wait while we load your content...