Coinage metal metallacycles involving a fluorinated 3,5-diarylpyrazolate†
Abstract
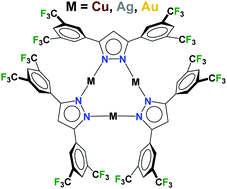
Copper(I) and silver(I) pyrazolate complexes {[3,5-(3,5-(CF3)2Ph)2Pz]Cu}3 and {[3,5-(3,5-(CF3)2Ph)2Pz]Ag}3 have been synthesized using the fluorinated 3,5-(diaryl)pyrazole 3,5-(3,5-(CF3)2Ph)2PzH and copper(I) oxide and silver(I) oxide, respectively. The gold(I) analog was obtained from a reaction between Au(THT)Cl and [3,5-(3,5-(CF3)2Ph)2Pz]H/NaH. The X-ray crystal structures show that the coinage metal complexes {[3,5-(3,5-(CF3)2Ph)2Pz]M}3 (M = Cu, Ag, Au) are trinuclear in the solid state. They feature distorted nine-membered M3N6 metallacyclic cores. The M–N distances follow Cu < Au < Ag, which is the trend expected from covalent radii of the corresponding coinage metal ions. The 3,5-(3,5-(CF3)2Ph)2PzH forms hydrogen bonded trimers in the solid state that are further organized by π-stacking between aryl rings. Solid samples of {[3,5-(3,5-(CF3)2Ph)2Pz]M}3 display blue photoluminescence. The copper complex {[3,5-(3,5-(CF3)2Ph)2Pz]Cu}3 is an excellent catalyst for mediating azide–alkyne cycloaddition chemistry.


 Please wait while we load your content...
Please wait while we load your content...