Phosphine-catalyzed Michael additions to α-methylene-γ-butyrolactones†
Abstract
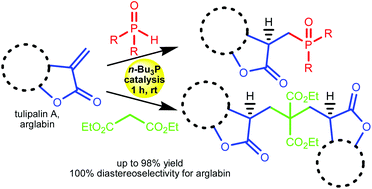
The highly efficient addition of phosphorus and carbon pronucleophiles to α-methylene-γ-butyrolactones (tulipalin A and arglabin) under n-Bu3P catalysis is reported. Kinetic experiments indicate that the unprecedentedly high reactivity of α-methylene-γ-butyrolactones results from the rigid s-cis geometry of the 1-oxa-1,3-butadiene moiety that favors generation of zwitterionic intermediate stabilized by interaction between the phosphonium center and adjacent carbonyl oxygen. The presented strategy offers an economical and practical method for functionalization of natural biologically active α-methylene-γ-butyrolactones with high levels of chemo- and stereoselectivity.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...