Acid catalysed rearrangement of isobenzofurans to angularly fused phthalides†
Abstract
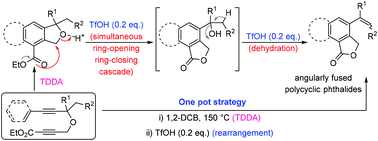
An acid catalysed, cascade process for the construction of angularly fused polycyclic phthalides from isobenzofurans under transition metal free conditions has been reported. This process is very general for diverse gem-disubstituted isobenzofuran substrates. Control experiments supported the mechanism as the nucleophilic attack of the carboxylate onto the acid activated furan ring for the simultaneous ring closing–ring opening cascade followed by dehydration. This method serves as a greener alternative for the synthesis of angularly fused polycyclic phthalides.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...