Ru(ii)-Catalysed synthesis of (1H)-isothiochromenes by oxidative coupling of benzylthioethers with internal alkynes†‡
Abstract
The synthesis of 1H-isothiochromenes by oxidative coupling of benzyl(tert-butyl)thioethers with internal alkynes, catalysed by Ru(II), has been achieved. The reaction occurs by S-directed C–H activation at the ortho position of the aryl ring, promoted by ruthenium, migratory insertion of the alkyne, 1,2-thio-Wittig rearrangement of the tert-butyl group and reductive elimination by C–S coupling between the resulting anionic sulfide and the vinylic carbon.
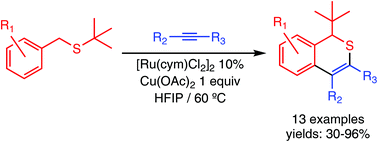
- This article is part of the themed collections: Synthetic methodology in OBC and Direct C-H Functionalization in Method Development and Late Stage Functionalization


 Please wait while we load your content...
Please wait while we load your content...