A new strategy for the synthesis of pyridines from N-propargylic β-enaminothiones†
Abstract
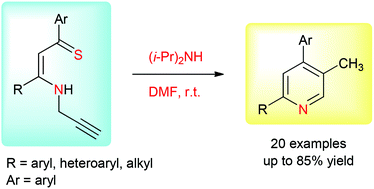
A new method for the synthesis of 2,4,5-trisubstituted pyridines from N-propargylic β-enaminothiones is reported. β-Enaminothiones were prepared by thionation of the corresponding β-enaminones with Lawesson's reagent. When treated with diisopropylamine in DMF at room temperature, N-propargylic β-enaminothiones yielded 2,4,5-trisubstituted pyridines in moderate to high yields, along with small amounts of 2-methylene-2,3-dihydro-1,4-thiazepines. The reaction was found to be general for a broad range of N-propargylic β-enaminothiones and tolerated the presence of aromatic, heteroaromatic and aliphatic groups with electron-withdrawing and electron-donating substituents. The method could be widened to the internal alkyne-tethered N-propargylic β-enaminothiones. This operationally simple method may provide rapid access to a library of functionalized pyridines of pharmacological interest.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...