New N-phenyl-4,5-dibromopyrrolamides as DNA gyrase B inhibitors†
Abstract
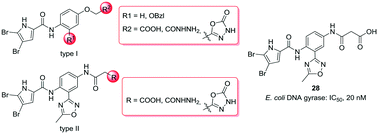
Due to the rapid development of antimicrobial resistance, the discovery of new antibacterials is essential in the fight against potentially lethal infections. The DNA gyrase B (GyrB) subunit of bacterial DNA gyrase is an excellent target for the design of antibacterials, as it has been clinically validated by novobiocin. However, there are currently no drugs in clinical use that target GyrB. We prepared a new series of N-phenyl-4,5-dibromopyrrolamides and evaluated them against DNA gyrase and against the structurally and functionally similar enzyme, topoisomerase IV. The most active compound, 28, had an IC50 of 20 nM against Escherichia coli DNA gyrase. The IC50 values of 28 against Staphylococcus aureus DNA gyrase, and E. coli and S. aureus topoisomerase IV were in the low micromolar range. However, the compounds evaluated did not show significant antibacterial activities against selected Gram-positive and Gram-negative bacteria. Our results indicate that for potent inhibition of DNA gyrase, a combination of polar groups on the carboxylic end of the molecule and substituents that reach into the ‘lipophilic floor’ of the enzyme is required.


 Please wait while we load your content...
Please wait while we load your content...