Synthesis, molecular docking, and biological evaluation of novel 2-pyrazoline derivatives as multifunctional agents for the treatment of Alzheimer's disease†
Abstract
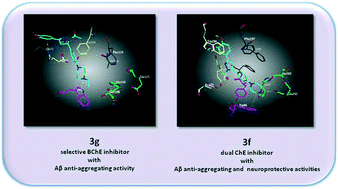
A novel series of 2-pyrazoline derivatives were designed, synthesized, and evaluated for cholinesterase (ChE) inhibitory, Aβ anti-aggregating and neuroprotective activities. Among these, 3d, 3e, 3g, and 3h were established as the most potent and selective BChE inhibitors (IC50 = 0.5–3.9 μM), while 3f presented dual inhibitory activity against BChE and AChE (IC50 = 6.0 and 6.5 μM, respectively). Kinetic analyses revealed that 3g is a partial noncompetitive inhibitor of BChE (Ki = 2.22 μM), while 3f exerts competitive inhibition on AChE (Ki = 0.63 μM). The active compounds were subsequently screened for further assessments. 3f, 3g and 3h reduced Aβ1–42 aggregation levels significantly (72.6, 83.4 and 63.4%, respectively). In addition, 3f demonstrated outstanding neuroprotective effects against Aβ1–42-induced and H2O2-induced cell toxicity (95.6 and 93.6%, respectively). Molecular docking studies were performed with 3g and 3f to investigate binding interactions inside the active sites of BChE and AChE. Compounds 3g and 3f might have the multifunctional potential for use against Alzheimer's disease.


 Please wait while we load your content...
Please wait while we load your content...