Salt loading in MOFs: solvent-free and solvent-assisted loading of NH4NO3 and LiNO3 in UiO-66†
Abstract
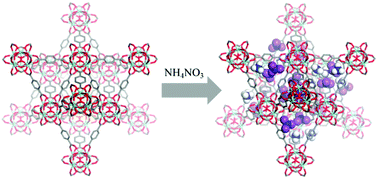
While the loading of liquid or solid materials in the pores of metal–organic frameworks (MOFs) can yield composite materials with novel and useful emergent properties, the loading of solids, and ionic solids in particular, can be challenging. We report the loading of the salts NH4NO3 and LiNO3 in the MOF UiO-66. The relatively low-melting NH4NO3 is loaded in UiO-66 in a solvent-free method, and loading is complete in 8 h at 75 °C, far below the melting point of NH4NO3. The higher-melting LiNO3 requires a small amount of solvent (water) for loading, and active removal of water assists in loading of the salt to form a composite that is 38% by mass LiNO3. These and similar salt–MOF composites are of interest for applications such as solid-ion conductors and energetic materials.


 Please wait while we load your content...
Please wait while we load your content...