Synthesis of polycyclic spirooxindoles via an asymmetric catalytic one-pot stepwise Aldol/chloroetherification/aromatization procedure†
Abstract
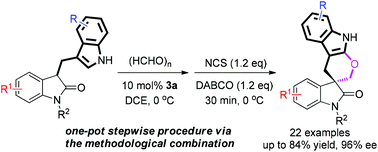
A general method for the synthesis of chiral pentacyclic spirooxindoles containing a tetrahydropyrano[2,3-b]indole scaffold through a one-pot stepwise sequence from 3-(3-indolomethyl)oxindole, paraformaldehyde and NCS is reported. Furthermore, the pentacyclic spirooxindoles could be transformed to bispirooxindole and other structurally diverse spirocyclic oxindoles.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...