Preparation of homoallylic amines via a three-component coupling process†
Abstract
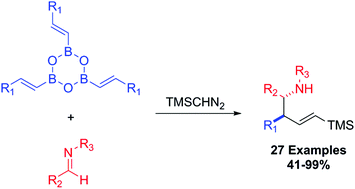
A three-component synthesis of homoallylic amines is described. The allylboronic species were generated in situ by homologation of vinyl boroxines with trimethylsilyldiazomethane, then followed by trapping of the allylboron intermediate with imines. Twenty-seven compounds were successfully prepared in moderate to high yields. Imines bearing various functional groups were tolerated, including aliphatic, aromatic and heteroaromatic substituents. Further elaboration of some of the homoallylic amines to form azeditines is also reported.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...