Effects of the ether oxygen atom in alkyl side chains on the physical properties of piperidinium ionic liquids†
Abstract
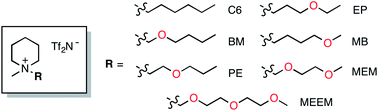
Various types of piperidinium ionic liquids (ILs) equipped with an oxygen atom-containing alkyl side chain on the positively charged nitrogen atom were systematically synthesized and their physical properties investigated. The thermal stability, viscosity, electrochemical window, and ion conductivity were influenced significantly by changing the position of the oxygen atom in the alkyl chain. Although the lowest viscosity was recorded for 1-((2-methoxyethoxy)methyl)-1-methylpiperidin-1-ium bis(trifluoromethylsulfonyl)amide ([PP1MEM][Tf2N]), 1-methyl-1-(2-propoxyethyl)piperidin-1-ium bis(trifluoromethylsulfonyl)amide ([PP1PE][Tf2N]) can be recommended as the best IL as an electrolyte due to its low viscosity and high thermal and electrochemical stability among the seven ILs tested.
- This article is part of the themed collection: Ionic liquids: from fundamental properties to practical applications


 Please wait while we load your content...
Please wait while we load your content...