Determining the composition of the vacuum–liquid interface in ionic-liquid mixtures†
Abstract
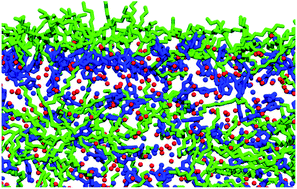
The vacuum–liquid interfaces of a number of ionic-liquid mixtures have been investigated using the combination of reactive-atom scattering with laser-induced fluorescence detection (RAS-LIF), selected surface tension measurements, and molecular dynamics (MD) simulations. The mixtures are based on the widespread 1-alkyl-3-methylimidazolium ([Cnmim]+) cation, including mixed cations which differ in chain length or chemical functionality with a common anion; and different anions for a common cation. RAS-LIF results imply that the surface compositions exhibit a general form of non-stoichiometric behaviour that mimics the well-known Henry’s and Raoult’s laws at low and high mole fraction, respectively. The extended Langmuir model provides a moderately good single-parameter fit, but higher-order terms are required for an accurate description. The quantitative relationship between RAS-LIF and surface tension, which probes the surface composition only indirectly, is explored for mixtures of [C2mim]+ and [C12mim]+ with a common bis(trifluoromethylsulfonyl)imide ([NTf2]−) anion. Extended Langmuir model fits to surface tension data are broadly consistent with those to RAS-LIF; however, several other common approaches to extracting surface compositions from measured surface tensions result in much larger discrepancies. MD simulations suggest that RAS-LIF faithfully reports on the alkyl-chain exposure at the surface, which is only subtly modified by composition-dependent structural reorganisation.
- This article is part of the themed collection: Ionic liquids: from fundamental properties to practical applications


 Please wait while we load your content...
Please wait while we load your content...