Stabilizing the OOH* intermediate via pre-adsorbed surface oxygen of a single Ru atom-bimetallic alloy for ultralow overpotential oxygen generation†
Abstract
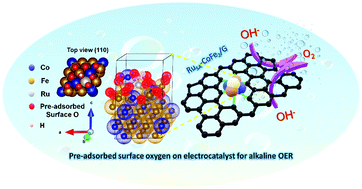
Designing efficient oxygen evolution reaction (OER) electrocatalysts based on single-atom catalysts is a highly promising option for cost-effective alkaline water electrolyzers. However, the instability of the OOH* intermediate and high energy barrier for the rate-determining step (RDS) (O* to OOH*) on the pure bimetallic-alloy represent serious challenges. Here, we report atomically dispersed Ru single-atoms on a cobalt–iron bimetallic-alloy encapsulated by graphitic carbon (RuSACoFe2/G) as an efficient and durable electrocatalyst for the alkaline OER. In-depth X-ray absorption spectroscopy (XAS) and aberration-corrected transmission electron microscopy (AC-TEM) along with theoretical calculations were employed to validate the isolated Ru sites in the surface-oxygen rich alloy. RuSACoFe2/G displays exceptional intrinsic activity, achieving a record low overpotential of only 180 mV at 10 mA cm−2 with superior durability in alkali media. Density functional theory (DFT) simulations revealed that the isolated Ru sites with pre-adsorbed surface oxygen species on a bimetallic-alloy efficiently stabilize the OOH* intermediate and significantly reduce the energy barrier for the RDS, boosting the intrinsic OER activity. Our integrated alkaline electrolyzer demands a low cell voltage of 1.48 V at 10 mA cm−2, suggesting that it has potential for use in practical applications.


 Please wait while we load your content...
Please wait while we load your content...