Thiolate–palladium(iv) or sulfonium–palladate(0)? A theoretical study on the mechanism of palladium-catalyzed C–S bond formation reactions†
Abstract
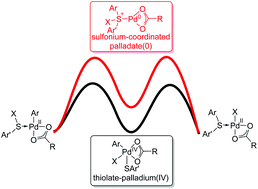
The density functional theory (DFT) method M06-L was used to study the general mechanism of palladium-catalyzed C–S bond formation reactions. Our theoretical calculations revealed that this type of reaction starts with a palladium-assisted metalation–deprotonation step. Oxidative addition of the sulfur source affords a thiolate–palladium(IV) intermediate, and subsequent reductive elimination generates the new C–S bond. A final protonation regenerates the active palladium(II) catalyst and releases the product. Our proposed mechanism could be applied to a series of palladium-catalyzed C–S bond formation reactions used for the construction of dibenzothiophene derivatives. The rate-limiting step of the catalytic cycle is oxidative addition to yield the thiolate–palladium(IV) intermediate. In contrast, formation of a sulfonium intermediate is unfavourable. In addition, the effect of substituents on the rate-determining step was studied with Hammett plots. Our calculations showed that incorporation of electron-withdrawing groups at the 4-position and electron-donating groups at the 15 and 16-positions would promote intramolecular oxidative addition of thioethers to palladium.
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2017

 Please wait while we load your content...
Please wait while we load your content...